Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 3
Cytoprotective Activity of Tribublus terrestris Dry Fruit Extract against Oxidative Stress
Pratik P Durgawale1*, Kailas D Datkhile1 and Satish V Kakade2
1Senior Research Officer, Molecular and Genetic Laboratory, Krishna Institute of Medical Sciences Deemed University, Karad, Maharashtra, India
2Associate Professor of Statistics, Department of Community Medicine, Krishna Institute of Medical Sciences Deemed University, Karad, Maharashtra, India
- *Corresponding Author:
- Pratik P Durgawale
Senior Research Officer
Molecular and Genetic Laboratory
Krishna Institute of Medical Sciences Deemed University
Karad, Maharashtra, India
Abstract
Oxidative stress generated in the cells has been reported in a number of diseases such as diabetes, cardiovascular disorders, neurodegenerative diseases M and cancer. Reactive oxygen species interact with almost all the biomolecules ultimately leading to cell death by apoptosis or necrosis. Since polyphenolic compounds such as flavanoids and tannins have radical scavenging activity, they could be effective in alleviating cellular damage caused by oxidative stress. The ethanolic extract of Tribublus terrestris dry fruit contains such polyphenolic compounds. In the present study oxidative stress was induced in neural cell line U-87 MG by exposure to hydrogen peroxide and effect on viability of cells was tested with 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) assay in presence or absence of the extract. Statistical analysis by Analysis of Variance (ANOVA) followed by Tukey-Kramer multiple comparison test (F=82.973, p<0.001) showed that the cells cultured with extract had greater viability compared to cells cultured without it in presence of hydrogen peroxide. DNA fragmentation assay was also performed which indicated that the extract could prevent apoptosis, characterized by fragmentation of chromosomal DNA and caused by oxidative stress. Further in-vivo experiments might have to be undertaken to ascertain the potential of the extract as a source of dietary anti-oxidants.
Keywords
Tribublus terrestris, Oxidative stress, U-87 MG
Introduction
Oxidative stress has been implicated in a myriad of diseases ranging from diabetes, cardiovascular diseases, neurodegenerative diseases and cancer [1-6]. Oxidative stress is generated at the cellular level mainly by strong oxidizing agents such as Reactive Oxygen Species (ROS), hydroxyl ions, oxygen free radicals, hydrogen peroxide, superoxide etc. These are produced in cells as intermediates or by-products of biochemical processes. If unattended, they interact with almost all of the biomolecules they come in contact with and lead to lipid peroxidation, complex formation with proteins, nucleic acids and finally cell death [7]. However, the cell has innate defense system against these free radicals such as redox glutathione system, superoxide dismutase, peroxidases, polyphenol compounds from diet which neutralize these ROS before they can cause any damage [8-11]. In physiological disorders, the amount of ROS produced in the cells exceeds the radical scavenging ability of this system and leads to further damage. Dietary source of polyphenolic compounds is a way of supplementing the anti- oxidative potential of the body and overcome the damage caused by ROS [12-15].
Tribublus terrestris, commonly called as goat thorn, belongs to Zygophyllaceae family which is an annual plant with fruits bearing thorn- like structures and grows mostly in warm conditions. This plant has been used in ethnomedicinal practices as an aphrodisiac, treatment of urinary tract infections, tonic, analgesic and diuretic [16]. In a previous study, the dry fruit extract of this plant was reported to contain the polyphenolic compounds flavanoids and tannins with radical scavenging activity [17]. In the present study, the cytoprotective activity of this extract was tested in a glioblastoma-derived cell line U-87 MG against hydrogen peroxide-induced oxidative stress using (3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide) (MTT) viability assay and DNA fragmentation assay.
Materials and Methods
T. terrestris dry fruits were purchased from a local Ayurvedic medicine shop. Spectrophotometer (Shimadzu) available at the Molecular and Genetic Laboratory was used. Other equipments and reagents used were procured as follows, hydrogen peroxide (Sisco Research Laboratories (SRL)), L-Ascorbic acid (SRL), ethanol (Merck). The neural cell line U-87 MG was obtained from National Centre for Cell Science, Pune. The cells were maintained in T-25 flasks (Nunc) containing Minimum Essential Medium (MEM, Gibco) supplemented with heat inactivated fetal bovine serum (FBS, Gibco), penicillin-streptomycin (Penicillin 100 units/ml, streptomycin 100 μg/ml) (Gibco). The flasks were incubated in 37°C, 5% CO2 humidified incubator (Thermo Scientific). Other instruments and reagents used were as follows: inverted microscope (Carl Zeiss) with Tucson H-series camera (Tucsen), haemocytometer (Hausser Scientific), 96-well plate (Nest), 3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide (MTT) reagent (Life technologies), 0.25% Trypsin 0.02% EDTA solution (Himedia), Dimethyl Sulfoxide (DMSO) (Loba Chemicals), Hanks Basal Salt Solution (HBSS, Gibco), Generuler 1 kbp DNA ladder (Thermo Scientific), Seakem LE agarose (Lonza), Sodium Dodecyl Sulphate (SDS) (SRL), sodium chloride (SRL), tris (SRL), isopropanol (Loba), ethanol (Chanshu Yangyuan Chemical).
Preparation of extract
The dry fruits of T. terrestris were powdered using mortar pestle and 10 g of powder was extracted using 200 millilitres (ml) ethanol in a Soxhlet extraction apparatus in four cycles. The extract was then dried and stored at –20°C until required. For assays, the dried extract was re-constituted in ethanol before use.
Cell line maintenance
The cell line U-87 MG was maintained in MEM supplemented with 10% FBS and antibiotics Penicillin-Streptomycin (Penicillin 100 units/ml, streptomycin 100 μg/ml) in T-25 culture flasks. At confluency, the flasks were passaged at a sub cultivation ratio of 1:2 to 1:3 with media renewal 2-3 times per week.
Viability assay
For testing viability of cells, the culture flasks at 70-80% confluency were passaged and 96-well plates were seeded at a density of 25,000 cells per well. The plates were then incubated in 37°C 5% CO2 incubator for 24 h. Depending on the assay being performed, the cells were then exposed to hydrogen peroxide, ascorbic acid, or extract along with appropriate controls for another 24 h. The media was then replaced with fresh medium and 0.5 mg/ml MTT and plates were incubated for another 2 h. Then media was carefully siphoned and DMSO was added to each well. After the formazan crystals had dissolved in DMSO, absorbance was measured at 540 nm and viability was calculated as follows:
% Viability = Abs540 of sample/Abs540 of cell control × 100
Where, Abs540 is the absorbance measured at 540 nm. Each assay was performed in triplicate in three independent experiments and the values represented henceforth are their mean values (n=9).
Cytotoxicity of T. terrestris extract
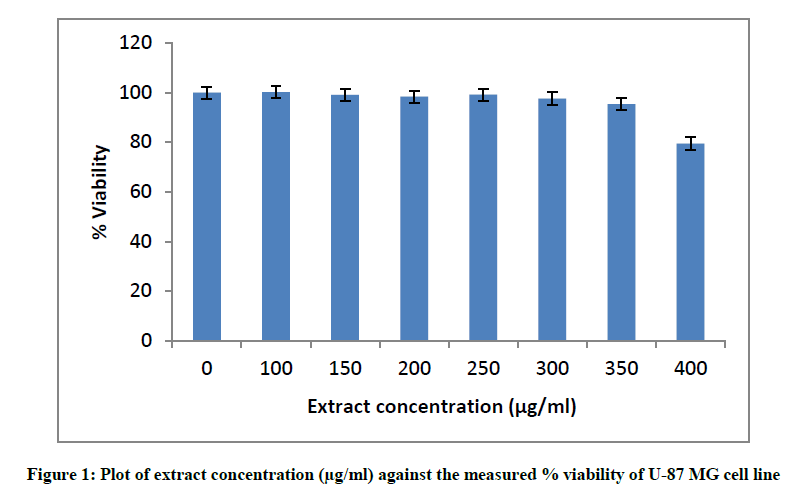
Initially, the cytotoxicity of T. terrestris extract was tested to determine the safe, non- toxic dosage of extract to be used for the U-87 MG cell line. The plates were seeded as mentioned and cells were exposed to varying concentrations of extract ranging from 10 μg/ml to 500 μg/ml for 24 h after which viability was assessed using MTT. Wells containing no extract served as the cell controls.
Cytotoxicity of hydrogen peroxide
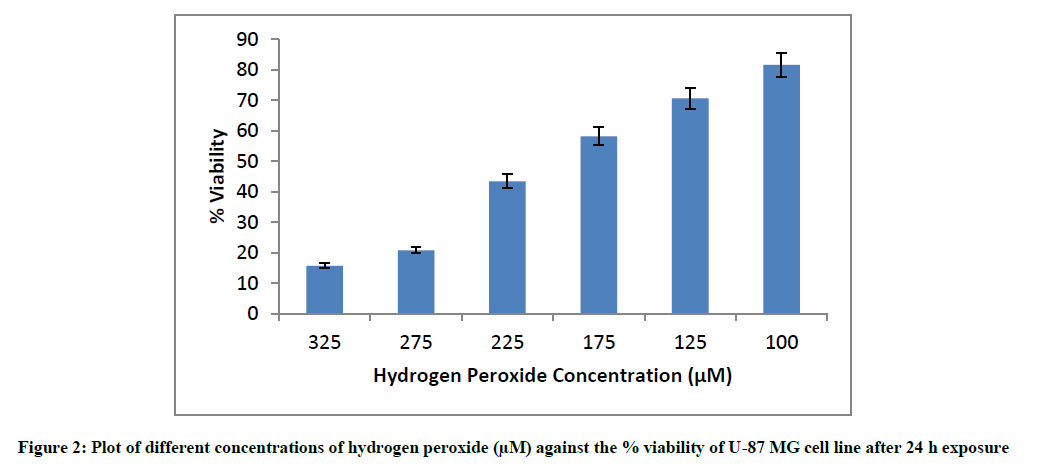
The plates were seeded for 24 h and cells were exposed to varying concentrations of hydrogen peroxide ranging from 100 μM to 325 μM for another 24 h, following which viability was measured using cells not exposed to hydrogen peroxide as the cell controls.
Cytoprotective activity of extract against induced oxidative stress
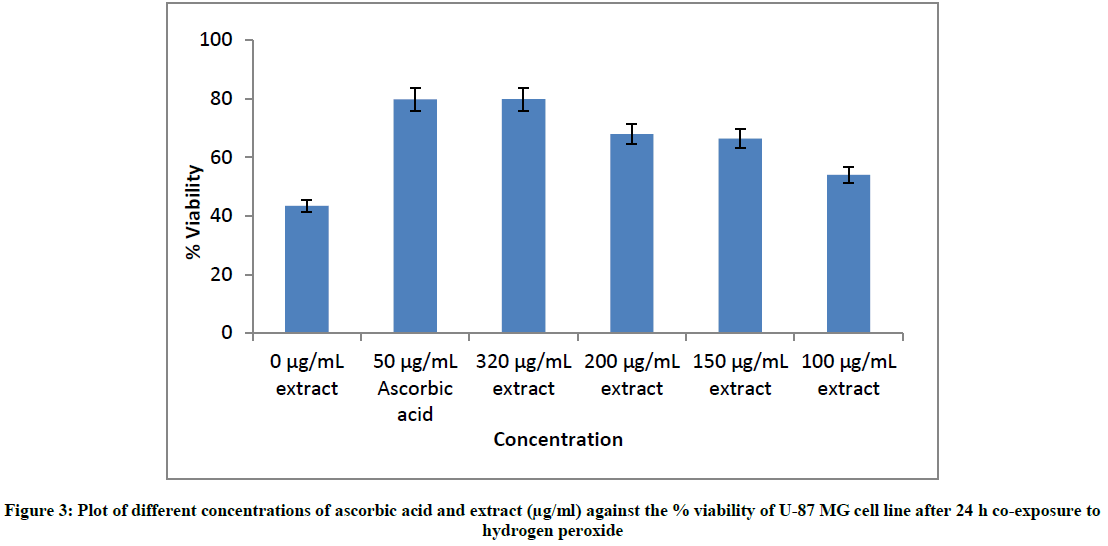
The plates were seeded for 24 h following which different non- toxic concentrations of the extract and 50 μg/ml ascorbic acid as positive control diluted in medium were added to respective wells. The plates were then incubated in 37°C, 5% CO2 incubator for 30 min. Hydrogen peroxide at a concentration of 225 μM was added to each set of wells i) Containing no extract or ascorbic acid, ii) Containing 50 μg/ml ascorbic acid, iii) Containing extract of concentration of 320 μg/ml, 200 μg/ml, 150 μg/ml and 100 μg/ml. Cell controls did not contain hydrogen peroxide, extract, or ascorbic acid. The plates were incubated for 24 h\s and viability was determined as mentioned.
Statistical analysis
To test the cytoprotective activity of the extract against hydrogen peroxide- induced oxidative stress, the observed values of % viability of cells exposure to hydrogen peroxide in the presence or absence of extract and ascorbic acid were analyzed using one-way Analysis of Variance (ANOVA) followed by Tukey- Kramer multiple comparison test. The analysis was done using Graphpad Instat software.
DNA fragmentation assay
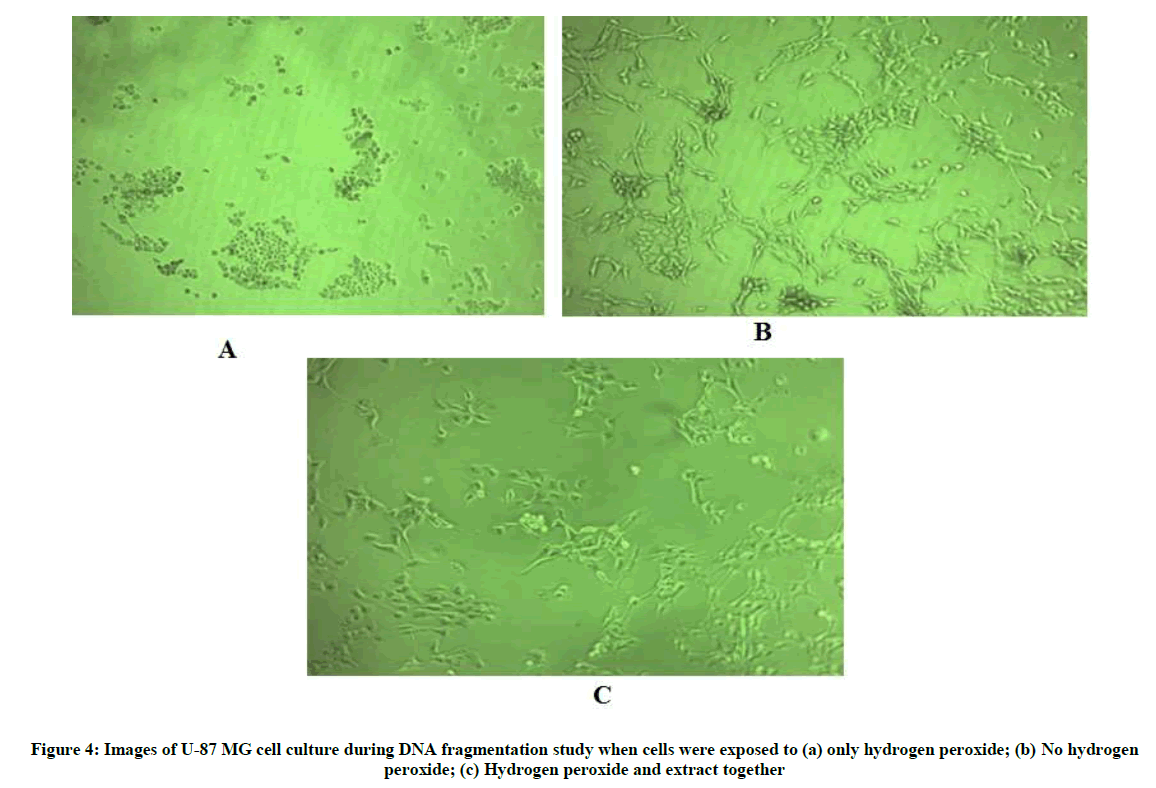
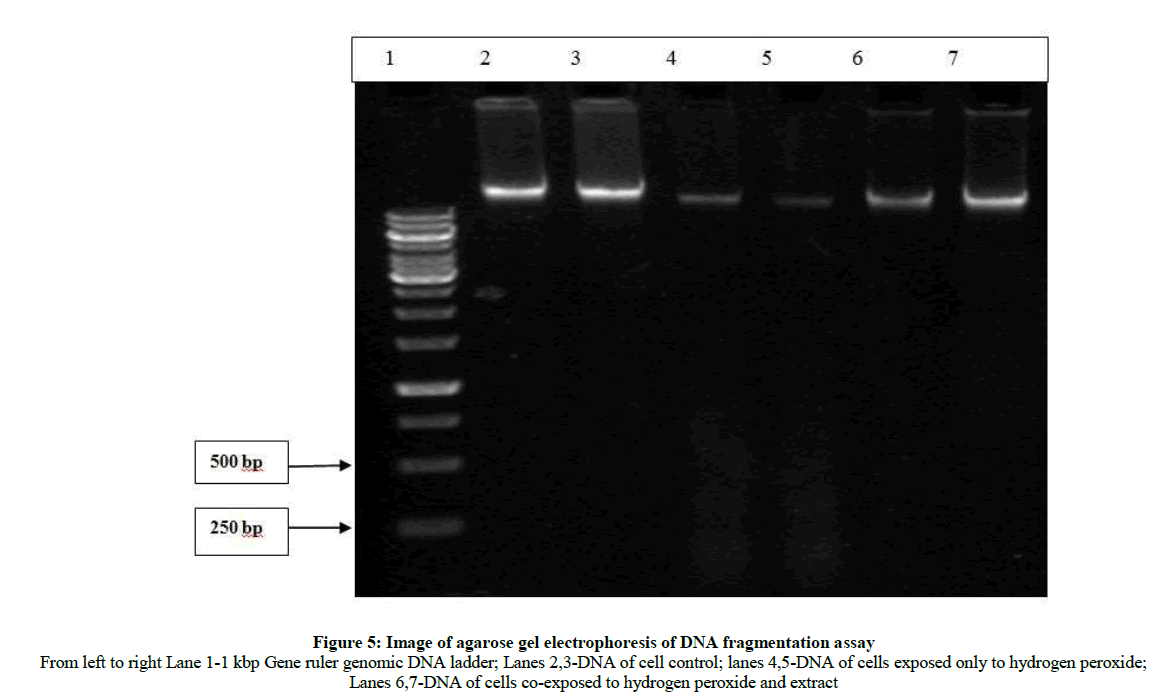
Since hydrogen peroxide is known to induce apoptosis in various cell lines, the U-87 MG cell line was also exposed to hydrogen peroxide and DNA fragmentation was studied [18-20]. 5 × 105 cells per well were seeded in 6-well plate for 24 h. Following which, the media was replaced with fresh medium and 320 μg/ml extract in medium was added to the test wells and plates were incubated for an additional 30 min. 250 μM hydrogen peroxide was added in wells except control wells which contained only freshly added medium and cells. Each well was treated in duplicate. The plate was incubated for 24 h. The cells were then harvested using Trypsin and washed twice with HBSS. Cells were then lysed in buffer containing 10 mM Tris-HCl, 1 mM EDTA, 0.2% triton X -100, 0.5% SDS. 0.5 mg/ml RNase A was added and incubated for 1 h at 37°C, thereafter proteinase K was added and incubated for 1 h at 55°C. DNA was then precipitated using sodium chloride and isopropanaol at -20°C. The suspension was the centrifuged and DNA pellet was washed with 70% ice-cold ethanol, air- dried and re-suspended in Tris-EDTA buffer (pH 8.0). The samples were run on 1.5% agarose gel.
Results and Discussion
Compounds with radical scavenging activity such as polyphenols can be a key component of the body’s defense mechanism against harmful ROS. Polyphenols are most notably present in fruits and vegetables. Hence, a proven dietary source of polyphenols can prevent damage to human health caused by ROS [21,22]. Curcumin, present in turmeric, has been proven to help against oxidative stress [23-25]. Similarly, consumption of citrus fruits, green tea and other medical plants have been reported to alleviate oxidative stress observed in various disorders and help overcome some of the disorder symptoms [26,12]. In a previous study, the ethanolic extract of T. terrestris dry fruits was found to contain polyphenolics and exhibited radical scavenging activity of free radicals of DPPH, ABTS, superoxide and ferric reducing activity [17]. To further evaluate the anti- oxidant potential of the extract, a glioblastoma cell line derived from the brain U-87 MG was used since neural cells are prone to damage caused by oxidative stress. Oxidative stress was induced by exposing the cells to hydrogen peroxide and different concentrations of extract were added to check if the viability of cells increases after incubation with extract. As a positive control, ascorbic acid was used as its activity as a radical scavenger is well documented [27,28].
Before beginning with the oxidative stress studies, the cytotoxicity of the extract itself was evaluated using the same cell line U-87 MG. The MTT assay used to measure cell viability relies on the principle of reduction of MTT to water- insoluble formazan crystals by succinate dehydrogenase present in active mitochondria of live cells. The absorbance of formazan crystals is then measured by dissolving it in DMSO and is proportional to the number of live cells [29]. After incubating the cells with different concentrations of the extract ranging from 100 μg/ml to 400 μg/ml, it was observed that the extract was cytotoxic at concentrations more than 350 μg/ml (Figure 1). The mentioned values of viability are mean percentage viability from three independent experiments.
To ascertain the hydrogen peroxide cytotoxicity in U-87 MG cell line, the cells were exposed to different concentrations of hydrogen peroxide for 24 h following which viability was measured. The experiment was done in triplicates in three independent trials (n=9). The values mentioned below are mean values of these experiments. Hydrogen peroxide is a strong oxidizing agent and is known to react with almost all the biomolecules. It brings about peroxidation of lipids and fats, forms complexes with proteins and nucleic acids [30]. The cytotoxicity of different concentrations of hydrogen peroxide against U- 87 MG cell line has been stated below. The % viability values are mean of three independent experiments (Figure 2).
In the next set of experiments, the cells were exposed to hydrogen peroxide in presence of extract and their viability was compared to cells exposed only to hydrogen peroxide. As positive control, cells were also exposed to ascorbic acid as it is a well-studied free radical scavenger with cytoprotective activity [27,28]. The % viability values stated below are mean of three independent experiments each performed in triplicate (Figure 3).
To analyse the data, one-way ANOVA was applied since the data fit the Gaussian distribution model. ANOVA revealed significant difference between cell control containing only hydrogen peroxide and cells exposed to ascorbic acid as well as different concentrations of the extract (F=82.973, p<0.001). The Tukey- Kramer multiple comparison tests indicated that the difference of mean values of % viability of ascorbic acid and different concentrations of extract compared to mean value of % viability of cells exposed only to hydrogen peroxide was significant (p < 0.001). In other words, viability of cells exposed to different concentrations of extract or ascorbic acid was significantly higher than those exposed only to hydrogen peroxide. This is indicative of presence of cytoprotective activity of the extract against hydrogen peroxide-induced oxidative stress in the neural cell line U-87 MG.
The DNA fragmentation assay helps to understand the mechanism of cell death as apoptosis is characterized by an activation cascade of proteases and enonucleases which ultimately lead to cleavage of chromosomal DNA. This fragmented DNA when analysed by agarose gel electrophoresis appears as a laddering of about 200 bp DNA [31]. In the present study, hydrogen peroxide was used to induce oxidative stress in the presence and absence of the extract. The images taken above (Figure 4) show the cell control which was not exposed to hydrogen peroxide as having live cells with clearly defined borders (Figure 4b), whereas the cells exposed only to hydrogen peroxide appear to be rounded, shrunken with irregular borders which is indicative of dead cells (Figure 4a). The cells co-exposed to hydrogen peroxide and extract (Figure 4c) appear to have clearly defined borders similar to cells not exposed to hydrogen peroxide indicating no observable adverse effect of hydrogen peroxide in presence of extract to the cells with respect to membrane integrity and appearance. The gel image below shows (from left to right) the cell control with intact genomic DNA in lanes 2 and 3, followed by DNA of cells exposed only to hydrogen peroxide in lanes 4 and 5 and DNA of cells co-exposed to hydrogen peroxide and extract in lanes 6 and 7 (Figure 5). It can be seen in lanes 4 and 5 that the genomic DNA appears to be fragmented in approximately 200 bp ladder-like form which is a trademark of apoptosis [31]. However, when the cells were co-exposed to extract, this fragmentation effect is not observed. This indicates that the extract has cytoprotective activity which prevents apoptosis of cells resulting from hydrogen peroxide-induced oxidative stress.
Conclusion
The obtained results indicate that the ethanolic extract of T. terrestris dry fruit protects cells from oxidative damage induced by hydrogen peroxide. This cytoprotective ability of the extract can be attributed to the presence of polyphenolic compounds having free radical scavenging activity. Further in vivo experiments may help better understand the anti-oxidant potential of the extract.
Acknowledgement
The authors would like to extend their gratitude towards Honorable Vice-Chancellor, KIMSDU and Directorate of Research, KIMSDU for supporting and guiding the project by providing the necessary equipments and facilities used during this project.
References
- N.R. Madamanchi, A. Vendrov, M.S. Runge, Arteriosclerosis, Thrombosis and Vascular Biology., 2005, 25(1), 29-38.
- N. A. Simonian, J.T. Coyle, Ann. Rev. Pharmacol. Toxicol., 1996, 36(1), 83-106.
- J.W. Baynes, Diabetes., 1991, 40(4), 405-412.
- S. Reuter, S.C. Gupta, M.M. Chaturvedi, B.B. Aggarwal, Free Radic. Bio. Med., 2010, 49(11), 1603-1616.
- B. Halliwell, Biochem. J., 2007, 401(1), 1-11.
- S. Toyokuni, K. Okamoto, J. Yodoi, H. Hiai, FEBS Lett., 1995, 358(1), 1-3.
- B. Halliwell, Biochem. Oxidative Stress., 2007, 1147-1150.
- F. Galli, Cardiovascular Disorders in Hemodialysis., 2005, 149, 240-260.
- B. Halliwell, Am. J. Med., 1991, 91(3), 14-22.
- B.P. Yu, Physiol. Rev., 1994, 74(1), 139-163.
- H. Sies, FEBS J., 1993, 215(2), 213-219.
- P.K. Vayalil, A. Mittal, Y. Hara, C.A. Elmets, S.K. Katiyar, J. Investig. Dermatol., 2004, 122(6), 1480-1487.
- M. Hokayem, Diabetes Care., 2013, 36(6), 1454-1461.
- C. Feillet-Coudray, T. Sutra, G. Fouret, J. Ramos, C. Wrutniak-Cabello, G. Cabello, J.P. Cristol, C. Coudray, Free Radic. Biol. Med., 2009, 46(5), 624-632.
- A.K. Fenercioglu, T. Saler, E. Genc, H. Sabuncu, Y. Altuntas, J. Endocrinol. Invest., 2010, 33(2), 118-124.
- P. Ody, The Complete Guide to Medicinal Herbs, D. Kimbersley, (Editors), London, 2000, 223.
- P.P. Durgawale, K.D. Datkhile, Int. J. Pharmacog. Phytochem. Res., 2017, 9(5), 716-721.
- E.R. Whittemore, T.L. Deryk, C.W. Cotman, Neuroreport., 1994, 5(12), 1485-1488.
- J.H. Jang, Y.J. Surh, Mutat. Res. Genet. Toxicol. Environ. Mutagen., 2001, 496(1), 181-190.
- T. Matsura, Free Radic. Res., 1999, 30(1), 73-83.
- K.B. Pandey, S.I. Rizvi, Oxid. Med. Cell Longev., 2009, 2(5), 270-278.
- L. Bravo, Nutr. Rev., 1998, 56(11), 317-333.
- R. Motterlini, R. Foresti, R. Bassi, C.J. Green, Free Radic. Biol. Med., 2000, 28(8), 1303-1312.
- Y. Fu, S. Zheng, J. Lin, J. Ryerse, A. Chen, Mol. Pharmacol., 2008, 73(2), 399-409.
- Wu, Y. Zhe, F. Gomez-Pinilla, Experimen. Neurol., 2006, 197(2), 309-317.
- C. Rekha, G. Poornima, M. Manasa, V. Abhipsa, J. Pavithra Devi, H T. Vijay KUMAR, T R. Prashith Kekuda, Chem. Sci. Trans., 2012, 1(2), 303-310.
- H.J. Heo, C.Y. Lee, J. Agric. Food Chem., 2004, 52(25), 7514-7517.
- K.M. Yoo, C.H. Lee, H. Lee, B. Moon, C.Y. Lee, Food Chem., 2008, 106(3), 929-936.
- G. Fotakis, J.A. Timbrell, Toxicol. Lett., 2006, 160(2), 171-177.
- M. Finnegan, J. Antimicrob. Chemother., 2010, 65(10), 2108-2115.
- Saraste, K. Pulkki, Cardiovasc. Res., 2000, 45(3), 528-537.