Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 3
Comparision of Anti-diabetic Activity of Plant Based and Synthetic Aryltetalin Derivatives
Chaitramallu M1*, Parvathi G R2, Sindhu GM3 and Ranjini P42Department of Botany, Bangalore University, Bangalore, India
3Department of Botany, JJS College for Arts, Commerce and Science, Mysuru, India
4Department of Biotechnology, Maharani′s Science College for Women, Mysuru, India
Chaitramallu M, Department of Chemistry, SDM and MMK Mahilamahavidyalaya, Mysuru, India, Email: malluchaithra88@gmail.com
Received: 16-Jan-2024, Manuscript No. Dpc-24-132411 ; Editor assigned: 19-Jan-2024, Pre QC No. Dpc-24-132411 (PQ); Reviewed: 02-Feb-2024, QC No. Dpc-24-132411; Revised: 01-Jun-2024, Manuscript No. Dpc-24-132411 (R); Published: 29-Jun-2024, DOI: 10.4172/0975-413X.16.3.314-320
Abstract
The aryltetralin compound podophyllotoxin was extracted from the roots of plant P. peltatum and analogues of aryltetralin were synthesized using tetralone as a starting material. They were synthesized by replacing 1, 3‐methylene dioxy ring with dimethoxy, hydroxy, methyl, chlorine and hydrogen and methoxy group. The structure of the final compounds was confirmed by 1H NMR, 13C NMR, mass spectra and elemental analysis data and the analogues were screened for anti-diabetic activity. It is noteworthy that, all the synthesized derivatives exhibits good anti-diabetic activity with respect to standard reference.
Keywords
Podophyllotoxin; Bromination; Reduction; Antidiabetic activity
Introduction
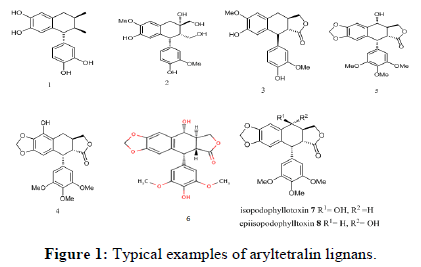
The aryltetralin compounds are secondary metabolites belongs to a class of natural products collectively referred to as lignans. The occurrence of lignans in nature is widespread and they have been shown to possess considerable diversity in their biological activity. Aryltetralin compounds have been found in a large number of species belonging to more than 60 families of vascular plants and have been isolated from different plant parts like roots, rhizomes, stems, leaves, fruits, seeds and from resins and exudates [1]. As such, there is a substantial interest in these compounds and their synthesis. Aryltetralin compounds in the plant kingdom has so far yielded over 200 lignans from nearly 70 different families, but the aryltetralin group has only been found in a few [2]. Nevertheless, as the result of continuing research, this number is increasing (Figure 1).
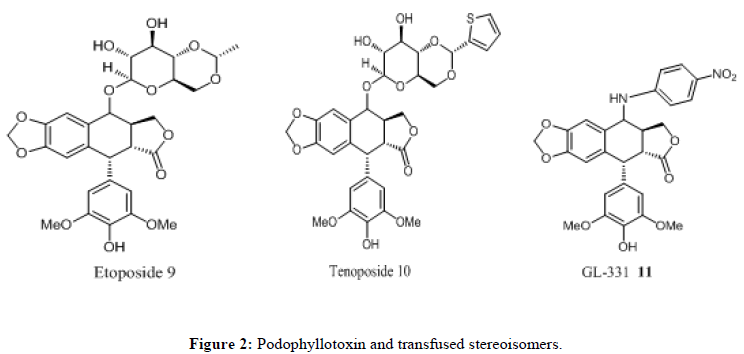
Among the aryltetralin group, there are two lignans which have a special significance. These are podophyllotoxin (5) and 4'- demethylpodophyllotoxin (6) their special importance lies in their anticancer activity [3]. These aryltetralin based compounds exhibit a wide variety of biological activities such as antimalarial, antifungal, antibacterial, antimitotic, anti-inflammatory, anticancer and anti-HIV(AIDS) [4]. There have been many synthetic modifications to the podophyllotoxin structure including the generation of the three potent anticancer agents 9, 10 and 11 (Figure 2). Etoposide (9) and teniposide (10) are DNA topoisomerase-II inhibitors presently in clinical use for the treatment of many cancers. However, drug resistance and various adverse drug reactions, including anemia, hair loss, severe gastrointestinal disturbances, hepatoxicity, immunosuppressant and neurologic symptoms etc; limited their clinical usages. So, continued efforts were still imperatively laid on the structural modification and pharmacological evaluation of aryltetralin derivatives to look for more potent agents. In addition, extensive Structure-Activity Relationship (SAR) studies have demonstrated that even a small alteration in the structures will cause significant change of their biological activities or even the molecular targets.
In view of the above facts, it was decided to modify the structure of podophyllotoxin (Figure 2).They were synthesized by replacing 1, 3‐methylene dioxy ring with dimethoxy, hydroxy, methyl, chlorine, hydrogen and methoxy group. The synthesized podophyllotoxin analogues were screened for their anti- diabetic assay [5-7].
Materials and Methods
All the reagents and chemicals were purchased from Merck chemicals used without further purification. Melting points were taken in open capillary tubes and are uncorrected. TLC is performed with E. Merck precoated silica gel plates (60F-254).Acme, India silica gel, 60-120 mesh is used for column chromatography. IR spectra in KBr were recorded on Perkin-Elmer model 683 spectrometers. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded using CDCl3 solvent containing Tetramethyl Silane (TMS) as internal references were recorded on bruker spectrometer. Elemental analyses were performed on a perkin-elmer 2400. Mass spectra were obtained by Water-Q-TOF ultima spectrometer. The root powder of P. peltatum for the extraction was obtained from botanical garden, botany department, Bangalore central university.
Extraction process
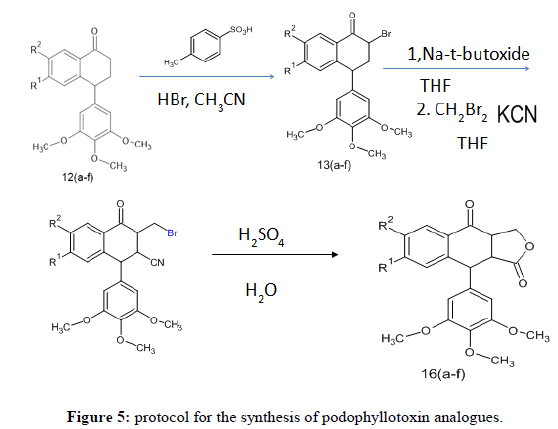
100gm root powder of P. peltatum was extracted with methanol (300 mL) in soxhlet apparatus for 6 hours. To the concentrated filtrate, added 200 ml water containing 2 mL HCl and cooled it. The mixture was allowed to stand for 5 hours below 50C under vacuum, washed with acidified water. Obtained residue was dissolved in 20 ml warm alcohol (90%). The obtained solution was filtered, evaporated to get podophyllotoxin. The structure was confirmed by spectroscopic techniques (Figure 3).
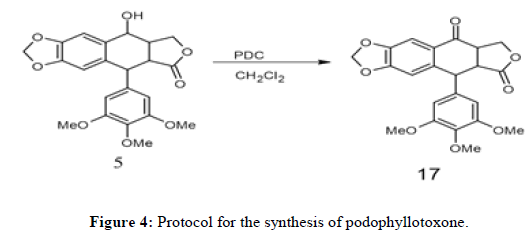
Conversion of podophyllotoxin to podophyllotoxone
General procedure for the synthesis of α-bromo derivative of 1-tetralone 3(a-f)
The starting material 2a-f (4 mmol) in acetonitrile (50 mL) and p-toluene sulphonic acid (1.14 g, 6 mmol) was heated at 65°C, N-bromosuccinimide (0.71 g, 4 mmol) was added slowly in lots over a period of 1 hour and the reaction mixture was stirred for 4-5 h. After completion of the reaction, reaction mixture was diluted with ethyl acetate (100 mL) and washed with water (2 × 50 mL). The ethyl acetate layer was separated and evaporated to get the crude product. The obtained product again recrystallized with ethanol to get a pure product.
2-bromo-6, 7-dimethoxy-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3a: Color: light brown solid; M.p 1680C; Yield: 75.18%: 1H NMR: 7.58-7.05 (2 H, s, Ar-H), 6.52(2 H, s, Ar-H), 4.93(1 H, t, CH-Br), 4.04 (1 H, t, J=4.7, CH), 3.80(15 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2); 13C NMR: 186.1, 154.7, 153.4, 147.2, 137.3, 136.7, 133.8, 127.3, 110.5, 109.2, 106.6, 60.8, 56.1, 53.9, 42.1, 39.9; MS, m/z: 452.15 (M+). Anal. Calcd. For C21H23BrO6: C, 55.89; H, 5.14; Br 17.70; O, 21.26 Found: C, 55.87; H, 5.04; Br 17.71; O, 21.29%.
2-bromo-6-hydroxy-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3b: Color: dark brown solid: M.p 1710C; Yield: 75.18%. 1H NMR: 8.15 (1 H, d, Ar-H), 7.12(1 H, d, Ar-H), 6.60-6.52(3 H, m, Ar-H),5.35(1 H, t, OH), 4.93(1 H, t, CH-Br), 4.04 (1 H, t, J=4.7, CH), 3.80(12 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2); 13C NMR: 186.1, 161.9, 153.4, 141.9, 137.3, 136.7, 130.7, 126.6, 120.6, 113.3, 106.6, 60.8, 56.1, 53.9, 42.1, 39.9;MS, m/z: 408.15 (M+). Anal. Calcd. For C19H19BrO5: C, 56.03; H, 4.70; Br 19.62; O, 19.64 Found: C, 56.07; H, 4.71; Br 19.62; O, 19.64%.
2-bromo-6-methyl-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3c: Color: dark brown solid. Yield: 65.18%. 1H NMR: 7.80- 7.13 (3 H, d, Ar-H), 6.52(2 H, s, Ar-H), 4.93(1 H, t, CH-Br), 4.04 (1 H, t, J=4.7, CH), 3.80(12 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2) 2.34 (3 H, s, CH3); 13C NMR: 186.1, 153.4, 143.3, 140.4, 137.3, 136.7, 131.0, 128.0, 126.4, 125.2, 106.6, 60.8, 56.1, 53.9, 42.1, 39.9; MS, m/z: 406.15 (M+). Anal. Calcd. For C20H21BrO4: C, 59.27; H, 5.22; Br 19.72; O, 15.79 Found: C, 59.28; H, 5.21; Br 19.76; O, 15.76 % (Figure 4).
2-bromo-6-chloro-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3d: Color: light brown solid. Yield: 71.18%. 1H NMR: 7.86-7.39 (3 H, q, Ar-H), 6.52(2 H, s, Ar-H), 4.93(1 H, t, CH-Br), 4.04 (1 H, t, J = 4.7, CH), 3.80(12 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2); 13C NMR: 186.1, 165.5, 153.4, 141.9, 139.2, 137.3, 136.7, 132.1, 130.7, 127.9, 126.2, 106.6, 60.8, 56.1, 53.9, 41.1, 39.9; MS, m/z: 426.15 (M+). Anal. Calcd. For C19H18BrClO4: C, 53.61; H, 4.26; Br 18.77; Cl, 8.33, O, 15.03 Found C, 53.60; H, 4.25; Br 18.79; Cl, 8.31, O, 15.07%.
2-bromo-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3e: Color: brown coloured solid. Yield: 67.78%. 1H NMR: 7.95-7.35 (6 H, m, Ar-H), 6.52(2 H, s, Ar-H), 4.93(1H, t, CH-Br), 4.04 (1H, t, J=4.7, CH), 3.80(12 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2); 13C NMR: 186.1, 153.4, 140.5, 137.3, 136.7, 134.0, 133.6, 128.1, 126.1, 106.6, 60.8, 56.1, 53.9, 41.8, 39.9; MS, m/z: 390.15 (M+). Anal. Calcd. For C19H19BrO4: C, 58.33; H, 4.89; Br 20.42; O, 16.36 Found: C, 58.34; H, 4.83; Br 20.45; O, 16.37 %.
2-bromo-6-methoxy-4-(3, 4, 5-trimethoxyphenyl)-3, 4-dihydronaphthalen-1(2H)-one 3f: Color: dark brown solid. Yield: 69.98%. 1H NMR: 8.25-6.89 (3 H, s, Ar-H), 6.52(2 H, s, Ar-H), 4.93(1 H, t, CH-Br), 4.04 (1 H, t, J=4.7, CH), 3.80(12 H, s, OCH3), 2.69-2.45 (2 H, m, J=6.4, CH2); 13C NMR: 186.1, 165.5, 153.4, 147.2, 137.3, 136.7, 133.8, 127.3, 110.5, 109.2, 106.6, 60.8, 56.1, 53.9, 42.1, 39.9; MS, m/z: 422.15 (M+). Anal. Calcd. For C20H21BrO5: C, 57.09; H, 5.04; Br 18.97; O, 18.99 Found: C, 57.07; H, 5.01; Br 18.99; O, 18.97 %.
General procedure for the preparation of 4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4h)-one 4(a-f)
A mixture of 3a-f (3 mmol) and Sodium-tertiary-butoxide (0.317 g, 3.3 mmol) in THF (25 mL) was refluxed at room temperature under nitrogen atmosphere for 10 hours. The progress of the reaction was monitored by TLC which showed the absence of starting material in the reaction mixture. After completion of the reaction, reaction mixture was poured into water (100 mL) and extracted with ethyl acetate (50 mL). The evaporation of the ethyl acetate layer gave crude product. This was purified by ethyl acetate/hexane (90: 10 v/v) on silica gel to get pure product.
6, 7-dimethoxy-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4a: Color: pale yellow solid; M.p. 118°C; Yield: 70.18%. 1H NMR: 7.21- 7.09 (2 H, d, Ar-H), 6.88 (1 H, d, α-CH), 6.86(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.74 (1 H, t, J=4.7, CH), 3.89(15 H, s, OCH3); 13C NMR: 183.7, 156.1, 153.4, 147.8, 139.7, 136.7, 136.2, 134.6, 131.9, 126.3, 113.8, 106.7, 104.3, 60.8, 56.1, 47.4; MS, m/z: 372.15 (M+). Anal. Calcd. For C20H22O6: C, 68.10; H, 5.94; O, 25.99 Found: C, 68.07; H, 5.91; O, 25.97%.
6, 7-dimethoxy-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4a: Color: pale yellow solid; M.p. 118°C; Yield: 70.18%. 1H NMR: 7.21- 7.09 (2 H, d, Ar-H), 6.88 (1 H, d, α-CH), 6.86(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.74 (1 H, t, J = 4.7, CH), 3.89(15 H, s, OCH3); 13C NMR: 183.7, 156.1, 153.4, 147.8, 139.7, 136.7, 136.2, 134.6, 131.9, 126.3, 113.8, 106.7, 104.3, 60.8, 56.1, 47.4; MS, m/z: 372.15 (M+). Anal. Calcd. For C20H22O6: C, 68.10; H, 5.94; O, 25.99 Found: C, 68.07; H, 5.91; O, 25.97 %.
6-hydroxy-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4b: Color: cream coloured solid; M.p. 128°C; Yield: 65.08%. 1H NMR: 7.61-7.10 (2 H, d, Ar-H), 6.86 (1 H, d, α-CH), 6.82(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 6.79 (1 H, d, Ar-H), 5.30(1 H, s, OH), 4.79 (1 H, t, J=4.7, CH), 3.89(9 H, s, OCH3); 13C NMR: 183.7, 163.3, 153.4, 140.0, 139.7, 136.7, 136.2, 134.6, 130.2, 125.6, 114.4, 113.9, 104.3, 60.8, 56.1, 47.4; MS, m/z: 342.15 (M+). Anal Calcd. For C18H19O5: C, 69.95; H, 5.57; O, 24.51 found: C, 69.97; H, 5.56; O, 24.50 %.
6-methyl-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4c: Color: cream coloured solid. Yield: 75.18%. 1H NMR: 7.65-7.23 (3 H, m, Ar- H), 6.89 (1 H, d, α-CH), 6.86(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.76 (1 H, t, J = 4.7, CH), 3.89(9 H, s, OCH3), 2.35(3 H, s, CH3); 13C NMR: 183.7, 153.4, 144.7, 139.7, 138.5, 136.7, 136.2, 134.6, 130.6, 130.3, 130.0, 127.0, 104.3, 60.8, 56.1, 47.4, 21.6; MS, m/z: 324.15 (M+). Anal. Calcd. For C20H20O4: C, 74.07; H, 6.21; O, 19.73 Found: C, 74.09; H, 6.19; O, 19.75%.
6-chloro-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4d: Color: light yellow coloured solid. Yield: 75.18%. 1H NMR: 7.71- 7.49 (3 H, m, Ar-H), 6.89 (1 H, d, α-CH), 6.86(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.86 (1 H, t, J=4.7, CH), 3.92(9 H, s, OCH3); 13C NMR: 183.7, 153.4, 140.7, 140.0, 139.7, 136.7, 136.2, 134.6, 131.8, 131.1, 128.5, 126.8, 104.3, 60.8, 56.1, 46.4; MS, m/z: 344.15 (M+). Anal. Calcd. For C19H17 ClO4: C, 66.19; H, 4.97; Cl, 10.28; O, 18.56 Found: C, 66.17; H, 4.99; Cl, 10.26; O, 18.58%.
4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4e: Color: cream coloured solid. Yield: 75.18%. 1H NMR: 7.71- 7.49 (3 H, m, Ar-H), 6.89 (1 H, d, α-CH), 6.86(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.86 (1 H, t, J=4.7, CH), 3.92(9 H, s, OCH3); 13C NMR: 183.7, 153.4, 139.7, 138.6, 136.7, 136.2, 135.0, 134.6, 133.0, 128.7, 127.9, 126.7, 104.3, 60.8, 56.1, 47.4; MS, m/z: 311.15 (M+). Anal. Calcd. For C19H18O4: C, 73.54; H, 5.85; O, 20.62 Found: C, 73.53; H, 5.86; O, 20.64%.
6-methoxy-4-(3, 4, 5-trimethoxyphenyl) naphthalen-1(4H)-one 4f: Color: cream coloured solid. Yield: 75.18%. 1H NMR: 7.61-7.20 (1 H, d, Ar- H), 6.99 (1H d Ar-H), 6.86 (1 H, d, α-CH), 6.82(1 H, d, β-CH), 6.46(2 H, s, Ar-H), 4.70 (1 H, t, J=4.7, CH), 3.79(12 H, s, OCH3); 13C NMR: 183.7, 166.9, 153.4, 139.7, 139.6, 136.7, 136.2, 134.6, 129.8, 125.3, 112.8, 112.3, 104.3, 60.8, 56.1, 47.4; MS, m/z: 342.15 (M+). Anal. Calcd. For C20H20O5: C, 70.57; H, 5.92; O, 23.50 found: C, 70.58; H, 5.91; O, 23.52%.
General procedure for the preparation of 3-bromomethyl-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2- carbonitrile 5(a-f)
Potassium cyanide (0.1625 g, 2.5 mmol) was added to the reaction mixture of compound 4a-f ( 2.5 mmol), tetra butyl ammonium bromide (0.08 g, 0.25 mmol) and dibromomethane (0.64 g, 3.75 mmol) which were stirred under nitrogen gas atmosphere at room temperature using dried THF solvent (25 mL) and refluxed for 5 hours at 600 C. After completion of the reaction which was monitored by TLC, the reaction mixture was evaporated to dryness and extracted with water (100 mL) followed by Diethyl ether (50 mL). The organic layer was separated and was evaporated to dryness. The obtained solid compound was purified by column chromatography on silica gel using hexane/ethyl acetate (80:20 v/v).
3-(bromomethyl)-6, 7-dimethoxy-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5a: Color: yellowish solid; M.p 152°C; Yield: 62.88%. 1H NMR: 8.20 (1 H, d, Ar-H), 7.13 (2 H, s, Ar-H), 6.52-6.60 (2 H, t, Ar-H), 5.32(1 H, s OH), 4. 07 (1 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 3.80(1 H, t, CH-CN), 3.58-3.33(3 H, m, CH2-Br), 2.35(3 H, s, CH3); 13C NMR: 199.1, 161.9, 153.4, 141.9, 137.3, 136.7, 130.7, 126.6, 120.6, 119.2, 113.3, 106.6, 60.8, 56.1, 49.0, 36.7, 30.4, 26.8; MS, m/z: 492.15 (M+). Anal. Calcd. For C23H24BrNO6: C, 56.34; H, 4.93; Br, 16.30; N, 2.86; O, 19.58 Found: C, 56.38; H, 4.92; Br, 16.31; N, 2.15; O, 17.92%.
3-(bromomethyl)-7-hydroxy-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5b: Color: buff coloured solid; M.p. 1640C. Yield: 63.18%. 1H NMR: 8.20 (1 H, d, Ar-H), 7.13 (2 H, s, Ar-H), 6.52-6.60 (2 H, t, Ar-H), 5.32(1 H, s OH), 4. 07 (1 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 3.80(1 H, t, CH-CN), 3.58-3.33(3 H, m, CH2-Br), 2.35(3 H, s, CH3); 13C NMR: 199.1, 161.9, 153.4, 141.9, 137.3, 136.7, 130.7, 126.6, 120.6, 119.2, 113.3, 106.6, 60.8, 56.1, 49.0, 36.7, 30.4, 26.8; MS, m/z: 447.15 (M+). Anal. Calcd. For C21H20BrNO5: C, 56.52; H, 4.52; Br, 17.90; N, 3.14; O, 17.97 Found: C, 56.58; H, 4.58; Br, 17.93; N, 3.15; O, 17.92%.
3-(bromomethyl)-7-methyl-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5c: Color: yellow coloured solid; M.p. 174°C; Yield: 75.18%; 1H NMR: 7.80 (1 H, d, Ar-H), 7.24-7.13 (2 H, d, Ar-H), 6.52 (2 H, s, Ar-H), 4. 05 (1 H, d, J=4.0, CH), 3.93(9 H, s, OCH3), 3.80(1 H, t, CH-CN), 3.58-3.33(2 H, m, CH2-Br), 3.4(1 H, q, CH), 2.35(3 H, s, CH3); 13C NMR: 199.1, 153.4, 143.3, 140.4, 137.3, 136.7, 131.0, 128.0, 126.4, 125.2, 119.2, 106.6, 60.8, 56.1, 49.0, 36.7, 30.4, 26.8, 21.6; MS, m/z: 432.15 (M+). Anal. Calcd. For C22H22BrNO4: C, 59.47; H, 4.99; Br, 17.98; N, 3.16; O, 14.47 Found: C, 59.48; H, 4.98; Br, 17.99; N, 3.15; O, 14.40%.
3-(bromomethyl)-7-chloro-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5d: Color: light yellow solid; M.p. 154°C; Yield: 75.18%; 1H NMR: 7.80 (1 H, d, Ar-H), 7.24-7.13 (2 H, d, Ar-H), 6.52 (2 H, s, Ar-H), 4. 05 (1 H, d, J=4.0, CH), 3.93(9 H, s, OCH3), 3.80(1 H, t, CH-CN), 3.58-3.33(2 H, m, CH2-Br), 3.4(1 H, q, CH), 2.35(3 H, s, CH3); 13C NMR: 199.1, 153.4, 143.3, 140.4, 137.3, 136.7, 131.0, 128.0, 126.4, 125.2, 119.2, 106.6, 60.8, 56.1, 49.0, 36.7, 30.4, 26.8, 21.6; MS, m/z: 432.15 (M+). Anal Calcd. For C22H22BrNO4: C, 59.47; H, 4.99; Br, 17.98; N, 3.16; O, 14.47 Found: C, 59.48; H, 4.98; Br, 17.99; N, 3.15; O, 14.40%.
3-(bromomethyl)-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5e: Color: Dark yellow coloured solid; M.p. 169° C; Yield: 75.18%; 1H NMR: 7.92-7.35 (4 H, m, Ar-H), 6.56(2 H, s, Ar-H), 4. 0 (1 H, d, J=4.0, CH), 3.92(9 H, s, OCH3), 3.78(1 H, t, CHCN), 3.58-3.33(2 H, m, CH2-Br), 3.4(1 H, q, CH); 13C NMR: 199.1, 153.4, 140.5, 137.3, 136.7, 134.0, 133.6, 128.1, 126.1, 119.2, 106.6, 60.8, 56.1, 49.0, 36.4, 30.4, 26.8; MS, m/z: 432.15 (M+). Anal. Calcd. For C21H20BrNO4: C, 58.62; H, 4.68; Br, 18.57; N, 3.26; O, 14.87 Found: C, 58.65; H, 4.65; Br, 18.58; N, 3.25; O, 14.89%.
3-(bromomethyl)-7-methoxy-4-oxo-1-(3, 4, 5-trimethoxyphenyl)-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile 5f: Color: Dark yellow coloured solid; M.p. 179°C; Yield: 75.18%; 1H NMR: 7.88-7.39 (3 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 08 (1 H, d, J=4.0, CH), 3.82(9 H, s, OCH3), 3.76(1 H, t, CH-CN), 3.58-3.33(2 H, m, CH2-Br), 3.45(1 H, m, CH); 13C NMR: 199.1, 153.4, 141.9, 139.2, 137.3, 136.7, 132.1, 130.7, 127.9, 127.9, 119.2, 106.6, 60.8, 56.1, 49.0, 35.9, 30.4, 26.8; MS, m/z: 466.15 (M+). Anal Calcd. For C21H19BrClNO4: C, 54.27; H, 4.12; Br, 17.19; Cl, 7.63; N, 3.01; O, 13.77 found: C, 54.24; H, 4.11; Br, 17.18; Cl, 7.63; N, 3.05; O, 13.78%.
General procedure for the synthesis of 9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6(a-f)
To a stirred solution of compound 5a-f (0.980 g, 2 mmol) 20% sulphuric acid was added and refluxed at 900 C for 15 hours. After completion of the reaction, the reaction mixture was poured into ice, extracted with ethyl acetate and then ethyl acetate was evaporated to get crude solid which was purified by column chromatography on silica gel using hexane/ethyl acetate (80: 20v/v).
6,7-dimethoxy-9-(3,4,5-trimethoxyphenyl)-3,3a,9,9a-tetrahydronaphtho[2,3-c]furan-1,4-dione 6a: Color: white coloured solid; M.p. 151°C; Yield: 68.98%; IR: 1635 cm-1(benzylic C=O), 1755 cm-1 (lactone C=O);1H NMR: 7.98-7.35 (4 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 68 (2 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 4.58-3.59 (6 H, t, CH2); 13C NMR: 193.3, 175.4, 153.4, 140.5, 136.7, 136.1, 133.6, 134.0, 128.1, 126.1, 106.6, 69.3, 60.8, 56.1, 47.0, 46.7, 43.5; MS, m/z: 369.15 (M+). Anal Calcd. For C21H20O6: C, 68.47; H, 5.47; O, 26.06 Found: C, 68.49; H, 5.44; O, 26.08 %.
7-hydroxy-9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6b: Color: white coloured solid; M.p. 158°C; Yield: 68.09%; IR: 1675 cm-1(benzylic C=O), 1795 cm-1 (lactone C=O);1H NMR: 7.98-7.35 (4 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 68 (2 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 4.58-3.59 (6 H, t, CH2); 13C NMR: 193.3, 175.4, 153.4, 140.5, 136.7, 136.1, 133.6, 134.0, 128.1, 126.1, 106.6, 69.3, 60.8, 56.1, 47.0, 46.7, 43.5; MS, m/z: 369.15 (M+). Anal Calcd. For C21H20O6: C, 68.47; H, 5.47; O, 26.06 Found: C, 68.49; H, 5.44; O, 26.08 %.
7-methyl-9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6c: Color: white coloured solid; M.p. 167°C; Yield: 65.68%; IR: 1635 cm-1(benzylic C=O), 1755 cm-1 (lactone C=O); 1H NMR: 7.98-7.35 (4 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 68 (2 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 4.58-3.59; 13C NMR: 193.3, 175.4, 153.4, 140.5, 136.7, 136.1, 133.6, 134.0, 128.1, 126.1, 106.6, 69.3, 60.8, 56.1, 47.0, 46.7, 43.5; MS, m/z: 369.15 (M+). Anal Calcd. For C21H20O6: C, 68.47; H, 5.47; O, 26.06 Found: C, 68.49; H, 5.44; O, 26.08%.
7-chloro-9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6d: Color: white coloured solid; M.p. 161°C; Yield: 86.78%; IR: 1635 cm-1(benzylic C=O), 1755 cm-1 (lactone C=O); 1H NMR: 7.98-7.35 (4 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 68 (2 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 4.58-3.59 (6 H, t, CH2); 13C NMR: 193.3, 175.4, 153.4, 140.5, 136.7, 136.1, 133.6, 134.0, 128.1, 126.1, 106.6, 69.3, 60.8, 56.1, 47.0, 46.7, 43.5; MS, m/z: 369.15 (M+). Anal Calcd. For C21H20O6: C, 68.47; H, 5.47; O, 26.06 Found: C, 68.49; H, 5.44; O, 26.08%.
9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6e: Color: white coloured solid; M.p. 171°C; Yield: 75.78%; IR: 1675 cm-1(benzylic C=O), 1785 cm-1 (lactone C=O); 1H NMR: 7.98-7.35 (4 H, m, Ar-H), 6.58(2 H, s, Ar-H), 4. 68 (2 H, d, J=4.0, CH), 3.83(9 H, s, OCH3), 4.58-3.59 (6 H, t, CH2); 13C NMR: 193.3, 175.4, 153.4, 140.5, 136.7, 136.1, 133.6, 134.0, 128.1, 126.1, 106.6, 69.3, 60.8, 56.1, 47.0, 46.7, 43.5; MS, m/z: 369.15 (M+). Anal Calcd. For C21H20O6: C, 68.47; H, 5.47; O, 26.06 Found: C, 68.49; H, 5.44; O, 26.08 %.
7-methoxy-9-(3, 4, 5-trimethoxyphenyl)-3, 3a, 9, 9a-tetrahydronaphtho [2, 3-c] furan-1, 4-dione 6f: Color: white coloured solid; M.p. 167°C; Yield: 95.28%; IR: 1635 cm-1(benzylic C=O), 1755 cm-1 (lactone C=O); 1H NMR: 8.21-8.30 (1 H, d, Ar-H), 7.16(1 H, s, Ar-H), 7.05-7.07(1 H, d, Ar-H), 6.70(2 H, s, Ar-H), 4. 70- 4.48 (4 H, m, Ar-CH), 3.83(12 H, s, OCH3), 3.65-3.63 (4 H, t, CH2); 13C NMR: 193.7, 175.0, 165.5, 153.4, 149.3, 136.7, 136.1, 130.3, 126.3, 111.7, 106.6, 104.6, 69.3, 60.8, 56.1, 55.8, 46.7, 44.1, 43.5; MS, m/z: 398.45 (M+). Anal. Calcd. For C22H22O7: C, 6.32; H, 5.57; O, 28.11 Found: C, 66.33; H, 5.59; O, 28.09% (Figure 5).
Results and Discussion
Synthesis
Bromination of the compound 2(a-f) was carried out in acetonitrile using N-bromo succinimide as the brominating agent. The conditions adopted are a modification of the reported procedure. The bromination happened selectively at the α-position to the carbonyl group of the tetralone. The selective bromination of the compound 2(a-f) was performed using N-bromosuccinimide in acidic medium by maintaining optimum temperature of 70°C in acetonitrile solvent. Using of N-bromosuccinimide is to activate the p-toluene sulphonic acid at the carbonyl oxygen to facilitate the formation of bromonium ions that leads to the formation of α-bromo tetralones. Single time addition of NBS resulted in the formation of more dibromo derivative. An excess of NBS also resulted in the formation of dibromo derivative. Hence one equivalent of NBS was added slowly in lots. The progress of the reaction was monitored by TLC. The impurities were removed during crystallization. The reaction mixture after the completion of the reaction was washed with water and extracted with ethyl acetate. The ethyl acetate layer was evaporated to get the product which was recrystallized from 10% ethanol in hexane to give the product. The products were identified using 1H and 13C-NMR. 1H NMR showed characteristic triplet at δ 4.7 ppm-4.93 ppm for hydrogen at the carbon bearing the bromo group. The tertiary hydrogen at γ-carbon to the carbonyl showed a triplet at δ 4.05 ppm-4.48 ppm. 13C-NMR showed carbonyl carbon at δ 186.10 ppm. The mass spectrums of M-1, M+ 1 peak are observed in the ratio of 50.5: 49.5 indicating the presence of bromine atom in the compound.
The brominated tetralone was dehydrohalogenated to the compound 4(a-f). The reaction mixture was refluxed using sodium tertiary butoxide in THF as a solvent for 10 hour under nitrogen atmosphere. Use of milder bases like triethyl amine and sodium carbonate led to lesser yield and incomplete reaction. Slight excess of sodium tertiary butoxide caused faster reaction but lesser yield after purification, the optimum amount of base was critical for the reaction. Hence THF was found to be the ideal solvent for the reaction. The product was identified by 1H-NMR and 13C-NMR and mass spectra and IR spectra. Mass spectra showed absence of peaks associated with bromine atom. The 1H-NMR showed signal at δ 6.8-7.1 ppm for the protons across the double bond. The absence of multiplet also at δ 2.6 ppm also confirms the formation of the product.
Potassium cyanide solution was added to the mixture of dibromomethane and tetra-n-butylammonium bromide in dry THF under nitrogen atmosphere which was kept at 60°C. The progress of the reaction was monitored by TLC. The products were identified by 1H-NMR and mass spectra. 1H-NMR did not show signals at δ 6.8 ppm indicating the absence of double bond protons. It showed a doublet at δ 3.5 ppm for the protons on carbon bearing the bromine atom. 13C-NMR shows signals at δ 119.3 ppm indicates the presence of the cyanide group. The carbonyl group is intact at δ 200.4 ppm.
The 3-bromomethyl-1-phenyl-1, 2, 3, 4-tetrahydronaphthalene-2-carbonitrile was added to 20% sulphuric acid and refluxed for 15 hours. The reaction mixture was poured into ice and extracted with ethyl acetate. The crude compound is purified by column chromatography on silica gel using ethyacetate/hexane (80: 20). IR spectra showed two strong carbonyl absorptions, one at 1755 cm-1 attributed to the lactone ring and the other at 1635 cm-1 attributed to a benzylic carbonyl group. 1H-NMR of the compound shows signals of doublet at δ 4.5 ppm and at δ 4.7 ppm indicating the protons at C-1 and C-9. Multiplets are seen at δ 3.5 ppm which indicates the protons on the carbon in the tetrahydro naphthalene ring system.
13C-NMR shows signals as singlet at δ 176 ppm pertaining to the carbonyl group of the lactone moiety while the carbonyl on the tetralone ring shows as singlet at δ 200.7 ppm [11].
Anti-diabetic activity
The new synthetic analogues were screened for antidiabetic activity by determining the ability of compounds to inhibit alpha amylase by DNS method and by measuring the non enzymatic albumin glycosilation. In this study, amylase activity for synthesized compounds was assayed according to Bernfeld method (Bernfeld 1955). This assay is based on the oxidation of ketone functional group in synthesized compounds. The principal involved is the test for the presence of free carbonyl group (C=O), the so called reducing sugar. One mole of sugar will react with one mole of 3, 5-dinitrosalicylic acid. Simultaneously 3, 5-Dinitrosalicylic Acid (DNS) is reduced to 3-amino, 5-nitro salicylic acid under alkaline conditions [11].
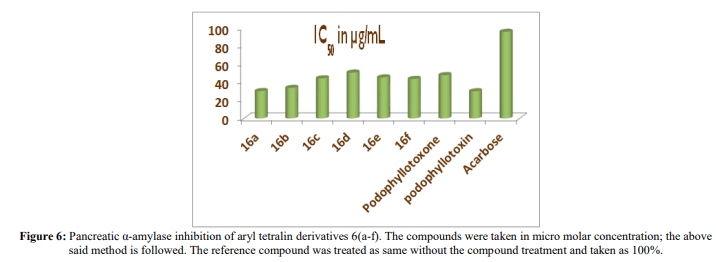
The pancreatic α-amylase inhibition of aryltetralin analogues 16(a-f) and podophyllotoxin, podophyllotoxone were studied in terms of oxidation of ketone functional group using DNS method. All the analogues inhibited α-amylase enzyme significantly with an IC50 values ranging between 30.27 µg/mL and 50.69 µg/mL compared to the reference compound acarbose with an IC50 value of 95.8 µg/mL presented in Table 1 (Figure 6).
| Compounds | IC50 in µg/mL |
|---|---|
| 16a | 30.27 |
| 16b | 33.79 |
| 16c | 44.37 |
| 16d | 50.69 |
| 16e | 45.38 |
| 16f | 43.7 |
| Podophyllotoxone | 47.89 |
| podophyllotoxin | 30.05 |
| Acarbose | 95.8 |
Table 1: Pancreatic α-amylase inhibition of aryltetralin derivatives 16(a-f), podophyllotoxone, podophyllotoxin.
Conclusion
In conclusion, the new aryltetralin analogues and the podophyllotoxone have been synthesized successively in good yield. The structures of synthesized compounds were confirmed and characterized by analytical data’s such as IR, 1H NMR, 13C NMR, Mass spectra and elemental analysis. They were screened for anti-diabetic assay and showed good alpha amylase inhibition activity compared to the parent compound of aryltetralin i.e., is podophyllotoxin.
References
- Madrigal B, Puebla P, Peláez R, et al. J Org Chem. 2003; 68(3): p. 854-864.
[Crossref] [Google Scholar] [PubMed]
- Reynolds AJ, Scott AJ, Turner CI, et al. J Am Chem Soc. 2003; 125(40): p. 12108-12109.
[Crossref] [Google Scholar] [PubMed]
- Andrews RC, Teague SJ, Meyers AI. J Am Chem Soc. 1988; 110(23): p. 7854-7858.
- Shivakumar SB, Basavaraju YB, Umesha B, et al. European J Chem. 2014; 5(3): p. 424-429.
- Sadashivamurthy B, Basavaraju YB. Bulg Chem Comm. 2005; 37(3):135-139.
- Umesha B, Basavaraju YB, Mahendra C. Med Chem Res. 2015; 24: p. 142-151.
- Ungurianu A, Şeremet O, Gagniuc E, et al. Pharmacolo Res. 2019; 150: p. 104522.
- Jackson DE, Dewick PM. Phytochemistry. 1985; 24(10): p. 2407-2409.
- Damayanthi Y, Lown JW. Curr Med Chem. 1998; 5: p. 205-252.
[Crossref] [Google Scholar] [PubMed]
- Haworth RD. J Chem Soc. 1942: p. 448-456.
- Chaitramallu M, Devaraju K, Dakshayini C, et al. Org Chem Curr Res. 2016; 5: p. 170.