Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 1
Comparative Evaluation of the Physicochemical and In Vitro Dissolution Properties of Some Expired and Unexpired Brands of Metformin Tablets
Jacob A Kolawole* and Bana DJNigeria
Jacob A Kolawole, Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Jos, Jos Plateau state, Nigeria, Email: kolajac@yahoo.com
Received: 07-Jan-2023, Manuscript No. dpc-23-87478; Editor assigned: 09-Jan-2023, Pre QC No. dpc-23-87478; Reviewed: 23-Jan-2023, QC No. dpc-23-87478; Revised: 24-Jan-2023, Manuscript No. dpc-23-87478; Published: 31-Jan-2023, DOI: 10.4172/0975-413X.15.1.21-29
Abstract
The comparative evaluation of the physicochemical properties and in vitro dissolution of some expired and unexpired brands of metformin tablets, six brands of expired (serial 1)and also unexpired (serial 2) of the same brand were used. The samples were determined using thin layer chromatography (TLC). Pharmacopoeia tests such as uniformity of weight, hardness, friability and assay were used to assess the physicochemical equivalence of the expired and unexpired metformin tablet brands. In vitro dissolution testing was conducted for both the expired and the unexpired tablets. All the brands (expired-serial 1) and (unexpired-serial 2) conformed to the British pharmacopoeia standard. All brands complied except C1 and F1 (within the range of not more than 1% (BP 2013) therefore brand C1 and F1 failed the friability test, the unexpired brand has higher crushing strength than the expired brands. Also for the assay content the unexpired brands has higher percentage content than the expired. The Dissolution test carried out, all the unexpired brands met the 70% drug released in 45 min (BP 2013) specification. The expired brands A1, D1, E1, F1 has lower percentage of drug released at 45 min, the unexpired brands has higher percentage of drug released at 45min compared to the unexpired. The thin layer chromatography (TLC) shows higher Rf value for the expired and there was an additional spot for the expired brands as compared to the unexpired brands.
Keywords
Physicochemical properties; Thin layer chromatography
INTRODUCTION
Diabetes mellitus is a group of metabolic diseases characterized by elevated levels of glucose in the blood (hyperglycemia) resulting from defects in insulin secretion, insulin action, or both (American Diabetes Association, 2003). There are two main types of diabetes mellitus (type 1 and type 2 diabetes mellitus) though there are other rare forms of diabetes mellitus. Type 1 diabetes (formerly known as insulin-dependent diabetes) is characterized by insulin deficiency resulting from pancreatic beta cell destruction. However, the most prevalent form of diabetes, type 2 diabetes (which accounts for over 90% of all diabetes cases) presents as a spectrum of metabolic abnormalities with prominent insulin resistance and relative insulin deficiency.
Drug dissolution testing is routinely used in the pharmaceutical industry to provide critical in vitro drug release information for both quality control i.e., to assess batch-to-batch consistency of solid oral dosage forms such as tablets and drug development, i.e., to predict in vivo drug release profile [1].
It serves as a quality control test in support of routine manufacture to establish batch-to-batch performance consistencies. Any substantial variations in the dissolution rate among the expired and the unexpired of same Brands indicate deterioration of the active ingredient. To this extent, manufacturing methods coupled with excipients used in the production, could contribute to the overall quality and release proficiency of medicament. Therefore, in order to ensure the requisite quality, drug manufacturers are required to test their products during and after manufacturing and at various intervals during the shelf life of the product [2].
It has been seen that expired brands of metformin are currently being sold out to patient in the market in Nigeria and other countries. Counterfeiting can apply to product and could include products which have expired. Also many drug sellers change the expiry date after the product have expired to enable the sales of such product, and also to validate or invalidate previous research reports in this area of study [3].
The work was done with the objectives.
To perform identification test on the various brands of metformin, both expired and unexpired brands of metformin tablets sampled using thin layer chromatography (TLC).
To determine the physicochemical equivalence of the expired and unexpired metformin tablet brands that would be sampled using both compendia and non- compendia method.
To assay the sampled metformin hydrochloride tablets using ultraviolent ray (UV) on both the expired and unexpired brands of metformin for comparison.
To perform a comparative in-vitro dissolution study of the expired and unexpired metformin tablets (Figure 1).
MATERIALS
Chemicals and Reagent
The chemicals used are, Glacial Acetic acid (Sigma- Aldrich, Germany), Butanol (Sigma- Aldrich, Germany), Ethanol (Sigma- Aldrich, Germany),Potassium dihydrogen orthophosphate (Kermel, china), and Sodium hydroxide pellets( Kermel, china).
Reagents
The reagents used are Absolute ethanol was obtained from African Centre of excellence in phytomedicine research and development(ACEPRD) university of Jos and Distill water was obtained from pharmaceutics department.
Equipments
Shimadzu UV.2650PC Double beam spectrophotometer [4], Electronic weighing balance (Baoshishan), Hardness tester.(DBK, India) , Friabilator (Erweka, Germany), Whatman filter paper, water bath was obtained from the department of pharmaceutical chemistry university of Jos.
Glass wares were used such as test tubes, cornical flask, measuring cylinder, 10ml pipette, ceramic mortar and pestle. pH Meter (Beckman model),and RC-6 Dissolution test apparatus 1L per vessel (Tianjin China).
Drugs
Standard metformin power was given by Prof. Ngwuluka, the commercial pharmaceutical formulations of Metformin were purchased from pharmacy stores within Jos. The brands are VPL-Metformin, Grakkophage, Gluformin, Diabetmin, Chanformin and Glyformin. The expired metformin of the same brand were given by my project supervisor, Prof. Jacob kolawole (Table 1) [5-7].
METHODS
Determination of Weight Variation
Twenty (20) tablets from above particular brand were randomly selected and the tablets were then weighed individually and the individual weight were sum up and averaged and the percentage deviation of each tablet from the mean was then calculated (British pharmacopoeia (2013). The procedure was repeated for the other brands both for the expired and unexpired brands.
Weight variation of each tablet was calculated individually for each tablet both for expired and unexpired metformin brands by using the formula:
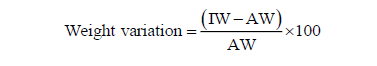
Where, IW = individual weight of tablet
AW= average weight of tablet
Hardness Measurement
Ten tablets were selected at random from each brand both expired and unexpired brands to perform this test using a hardness tester. A tablet was placed between the spindle and anvil of the tester and the calibrated scale adjusted to zero. Compression force was applied on the tablet and the position on the calibrated scale at which the tablet broke was recorded in kgf units. A mean hardness was calculated for each brand of both expired and the unexpired brands.
Friability Test
Ten tablets were randomly selected from above particular unexpired brand, dusted and weighed together and then placed in Erweka friabilator (Type TA 20, Germany) and operated for 4 minutes 25rpm.The tablets were dusted and reweighed and the percentage weight loss calculated. The procedure was repeated for other unexpired brands and same was done for the expired brands.
Extraction of Metformin Hydrochloride from Tablets
A quantity of the powdered tablets equivalent to 60mg of expired metformin hydrochloride was shaken with 60ml of ethanol and filtered with whatman filter paper (No 5). The filtrate was evaporated to dryness on a water bath and the residue dried at 105ºC for one hour (British pharmacopoeia, 2013). The procedure was repeated for the other expired brands and also for the unexpired brands and the residue of each brand used for thin layer chromatography analysis [8].
Identification of Extracted Metformin for both Expired and Unexpired Brands
Identification of extracted metformin using thin layer chromatography (TLC)
TLC silica gel G was used and the solvent system consisted of all mixture of glacial acetic acid, butanol and water in proportion of (10:40:50).20mg of the extracted residue of each unexpired metformin powder as well as 20mg of extracted residue of each expired metformin powder was dissolved in distilled water and diluted to 5ml with same solvent.
The test solution (the expired brand) and that of the reference (unexpired brands) were spotted on the plate and immersed in the solvent system contained in the chromatank. The plate was removed when the solvent had moved three -fourths of the length of the plate.
The solvent front was marked, allowed to evaporate from the plate and the spot detected by spraying with all mixture of equal volumes of sodium nitroprusside, potassium ferricyanide and sodium hydroxide solution all of 100g/L. The Rf value of each spot was then determined (British pharmacopoeia 2013) [9].
Invitro Dissolution Study
Preparation of phosphate buffer PH 6.8
Potassium dihydrogen orthophosphate solution was prepared by dissolving 272.18g of potassium dihydrogen orthophosphate in distilled water; this was made up to 30L using distilled water.
Sodium hydrogen solution was prepared by weighing 48g of sodium hydroxide pellets and dissolved in 4L of distilled water.
Sodium hydroxide solution was added to the 30L of potassium dihydrogen orthophosphate and the solution was then made up to 40L using distilled water.
The pH of the solution was taken to confirm the pH 6.8 of the phosphate buffer using the pH meter.
Preparation of standard calibration curve
A 0.1g quantity of metformin hydrochloride was weighed and dissolved in about 70ml of phosphate buffer PH 6.8, this was sonicated for five minutes with intermittent shaking. The solution was made up to 100ml using phosphate buffer PH 6.8. 10ml of the solution was taken and made up to 100ml to make a stock solution of 100μg/ml. Serial dilution was made from the stock to obtain working solution of 2μg/ml,4μg/ml,6μg/ml,8μg/ml, 10μg/ml.
- 0.2ml of stock was made up to obtain 2μg/ml
- 0.4ml of stock was made up to obtain 4μg/ml
- 0.6ml of stock was made up to obtain 6μg/ml
- 0.8ml of stock was made up to obtain 8μg/ml
- 1.0ml of stock was made up to obtain 10μg/ml
Dissolution test procedure
The sample test tubes were labelled with masking tape in order of media and time corresponding to each time point of withdrawal. The dissolution test was carried out using dissolution test apparatus with 6 vessels of 1litre capacity each and was conducted in two phases.
a. The first phase involves the use of the buffer solution and the different brands of Unexpired metformin tablet 500mg
b. The second phase involves the use of buffer solution and different brands of expired metformin tablet 500mg.
Both phases were carried out using phosphate buffer pH 6.8 at temperature of 37oC + 5oC at 50rpm. One tablet from each brand labelled were placed in basket/dissolution vessel and the machine was adjusted to start counting down from 60 minutes.
At 5, 10, 15,20,30,45 and 60 minutes, 5ml of dissolution sample were withdrawn and replaced with 5ml of dissolution medium immediately. The withdrawn samples were filtered with what man filter paper (No 5), also the blank (phosphate buffer 6.8) was filtered with what man filter paper.The filtrates were analyzed by UV spectrophotometer at 232nm.
The percentage drug released was calculated using,
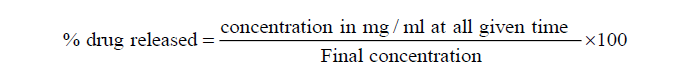
% drug released was plotted against time
The phosphate buffer 6.8 was used as the blank
Assay of Expired and Unexpired Brands of Metformin Using UV Analytical Method.
Stock Solution for Calibration Curve
A stock solution of metformin of concentration 0.1%w/v was prepared by dissolving 0.1g of pure metformin powder in small volume of distilled water and made up to 100ml. The following concentrations 0.1, 0.25, 0.3, 0.5, 0.7, 0.8 and 0.9μg/ml were then prepared from the stock solution.
The absorbance of these solutions was determined by ultraviolet spectrometry at all wavelength of 232nm. A calibration curve showing the relationship between concentration and absorbance was plotted and the equation and correlation values of the curve generated from the plot.
Assay Procedure
Tablets (20) of unexpired or expired metformin hydrochloride were weighed and powdered. A quantity of powder metformin tablets equivalent to 100mg of metformin hydrochloride was shaken with 70ml of distilled water for 15minutes, diluted to 100ml with water and filtered with what an filter paper (No5), discarding the first 20ml. 10ml of the filtrate was diluted to 100ml with distilled water, and 10ml of the resulting solution was further diluted to 100ml with distilled water. The absorbance of the resulting solution was measured at a wavelength of maximum absorption of 232nm. The content of metformin hydrochloride was then calculated using the calibration curve (British pharmacopoeia, 2013). Same procedure was carried out for the unexpired metformin brands [10,11].
RESULTS
Presentation of results
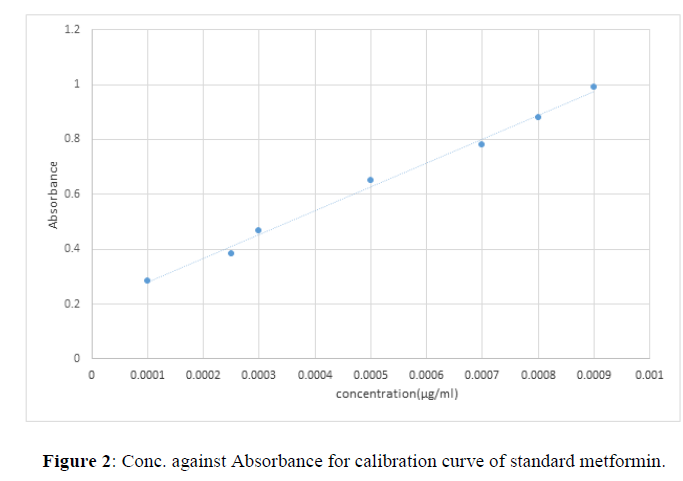
Table 1 shows the concentration and mean absorbance of standard metformin and Fig 1 shows the calibration curve for the standard metformin hydrochloride.
| CODE | NAFDAC NUMBER | BATCH NUMBER | MFG DATE | EXPIRY DATE | COUNTRY OF MFG |
|---|---|---|---|---|---|
| A1 | A4-2031 | 130708 | Jul-13 | Jul-16 | CHINA |
| A2 | A4-2031 | 170211 | Feb-17 | Feb-20 | CHINA |
| B1 | B4-0122 | GP14002 | Feb-14 | Sep-16 | INDIA |
| B2 | B4-0122 | 8145 | Apr-18 | Mar-21 | INDIA |
| C1 | Apr-26 | H0805 | Aug-13 | Aug-16 | NIGERIA |
| C2 | Apr-26 | PPK080118 | Oct-18 | Sep-21 | NIGERIA |
| D1 | 04-0810 | BE09410 | Sep-14 | Sep-17 | MALAYSIA |
| D2 | 04-0810 | BJ12591 | Dec-18 | Nov-21 | MALAYSIA |
| E1 | A4-6681 | 4J01 | Oct-14 | Sep-17 | INDIA |
| E2 | A4-6681 | 7F188 | Jun-17 | May-20 | INDIA |
| F1 | Apr-68 | S4307 | Jul-15 | Apr-17 | NIGERIA |
| F2 | Apr-68 | S4315 | Jul-17 | Jan-20 | NIGERIA |
| Note: all the ones with ‘1’ are Expired whereas all the ones with ‘2’ are unexpired | |||||
Table 2: Evaluated physicochemical properties of Expired and unexpired metformin hydrochloride tablet sample.
| Concentration (µg/ml) | Mean absorbance |
|---|---|
| 0.1 | 0.283 |
| 0.25 | 0.385 |
| 0.3 | 0.464 |
| 0.5 | 0.641 |
| 0.7 | 0.753 |
| 0.8 | 0.864 |
| 0.9 | 0.981 |
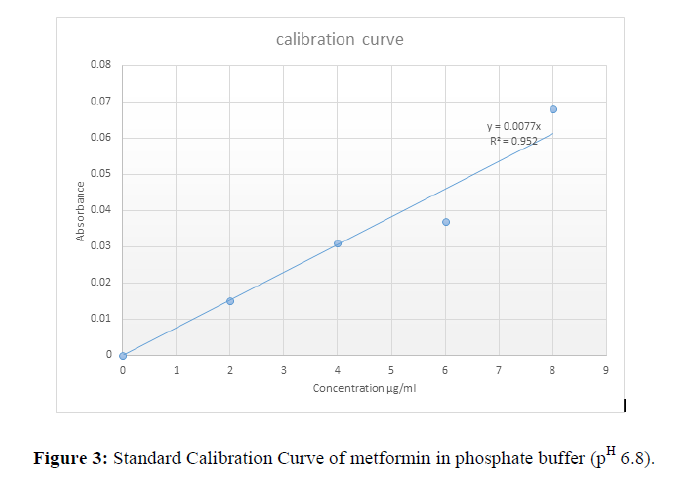
Table 3: Concentration and absorbance for dissolution calibration curve and Fig 2 Calibration Curve for dissolution test.
| Sample code | Mean weight(mg) | Mean Hardness(kgf) | % Friability | % Content | Mg content | Rf | Rf for additional spot | % Drug released |
|---|---|---|---|---|---|---|---|---|
| A1 | 643.5 | 8.26 | 0 | 86.94 | 434.7 | 0.52 | 0.93 | 64 |
| A2 | 618 | 11.04 | 0 | 98.6 | 493 | 0.51 | - | 98.8 |
| B1 | 692 | 2.35 | 0.146 | 96.61 | 483.05 | 0.51 | 0.92 | 70.8 |
| B2 | 604 | 5.55 | 0.99 | 98.6 | 493 | 0.49 | - | 97.5 |
| C1 | 547.5 | 10.24 | 1.08 | 84.15 | 420.75 | 0.52 | 0.94 | 76.4 |
| C2 | 541 | 12.16 | 0.19 | 100.01 | 500.05 | 0.48 | - | 95.1 |
| D1 | 565.5 | 5.15 | 0.18 | 84.45 | 422.25 | 0.51 | 0.94 | 69.8 |
| D2 | 534.5 | 8.17 | 0 | 100 | 500 | 0.47 | - | 94.7 |
| E1 | 603 | 6 | 0.166 | 80.66 | 403.3 | 0.55 | 0.94 | 69.7 |
| E2 | 604.5 | 7.75 | 0.165 | 101.4 | 507 | 0.48 | - | 87 |
| F1 | 542.5 | 7.05 | 5.75 | 83.55 | 417.75 | 0.5 | 69.9 | |
| F2 | 549 | 9.25 | 0.89 | 104.75 | 523.95 | 0.49 | 96.9 |
| Concentration µg/ml | Mean absorbance |
|---|---|
| 0 | 0 |
| 2 | 0.015 |
| 4 | 0.031 |
| 6 | 0.037 |
| 8 | 0.068 |
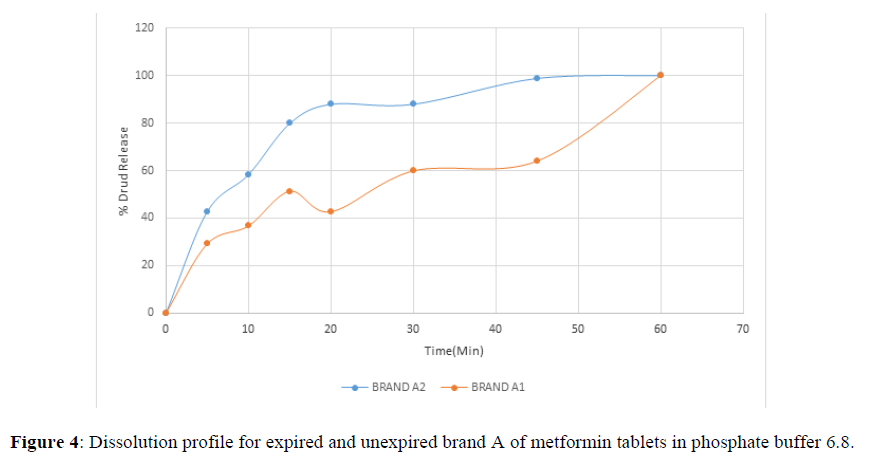
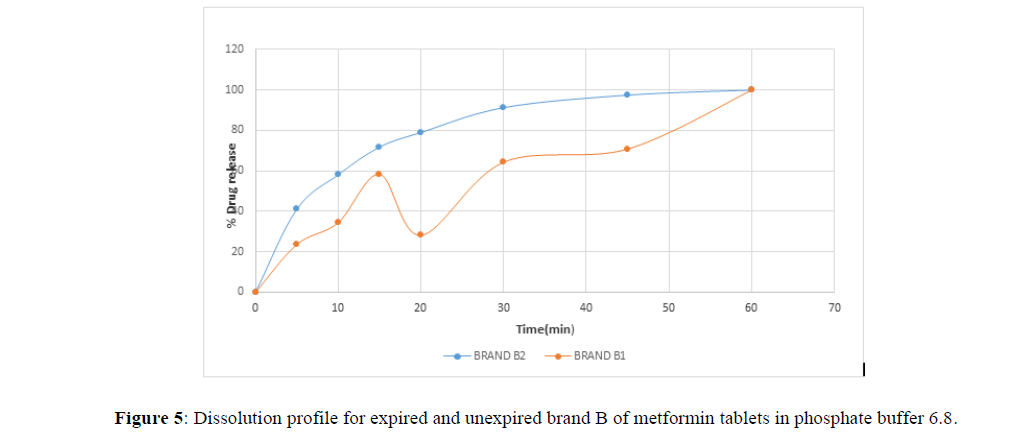
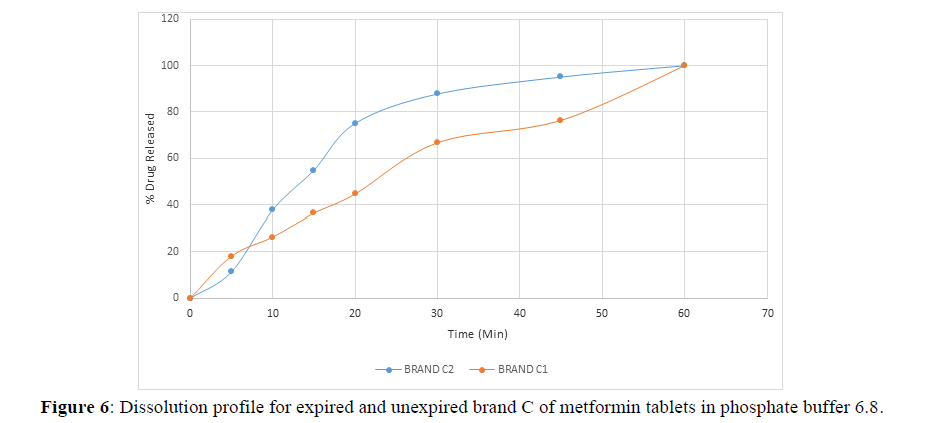
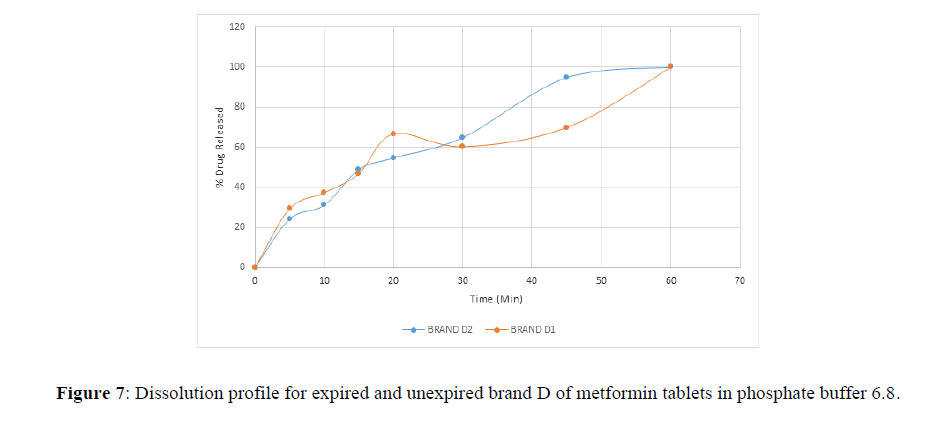
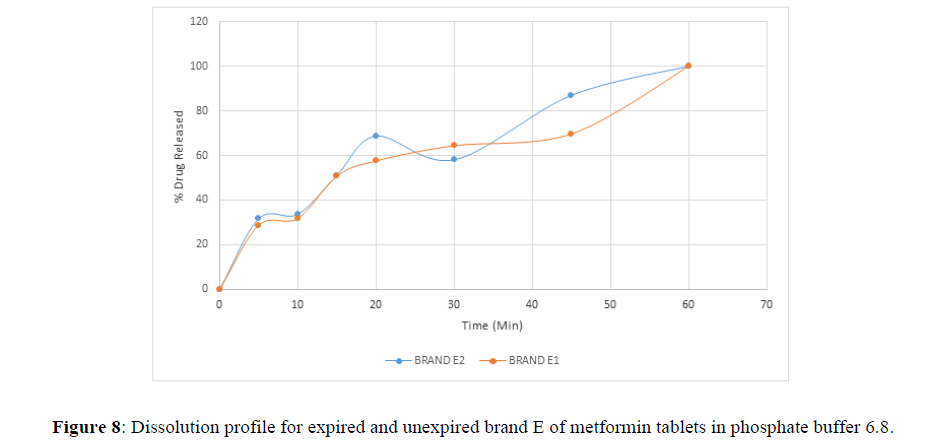
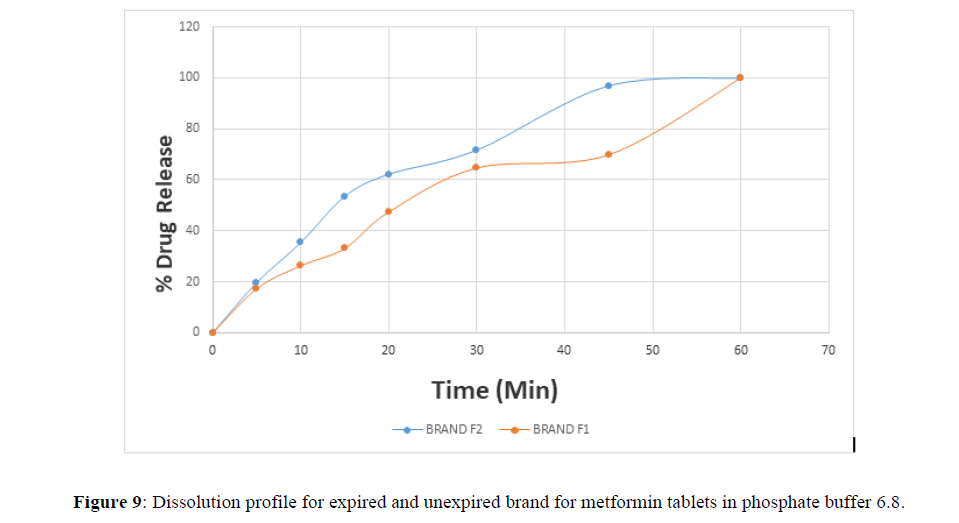
Fig 3,4,5,6,7,8,9 shows Dissolution profile for expired and unexpired brand A,B,C,D,E,F of metformin tablets in phosphate buffer 6.8.
Physicochemical properties of Expired and unexpired metformin hydrochloride tablet sample
Standard calibration curve of metformin in phosphate buffer (pH 6.8)
The concentration of standard metformin in phosphate buffer pH 6.8 was plotted against the various absorbance measured as shown in Fig 2.
DISCUSSION
Identification
Six brands of metformin hydrochloride both expired and unexpired brands were subjected to TLC analysis to ascertain the identity of the active pharmaceutical ingredient (API) and also to detect for any degraded product in the expired brands. Results from the identification test indicated that all the metformin tablets both expired and unexpired contained metformin hydrochloride as API; also the Rf for the TLC for the expired is higher than the unexpired and also the expired brands shows additional spot, which indicate presence of degraded products. The Rf value for the additional spots were calculated, showing the presence of degraded products.
Weight Uniformity
From table 2, all the brands(expired-serial 1) and (unexpired-serial2) conformed to the BP standard, not more than two of the individual weight of the expired and unexpired brands of metformin tablets should deviate from the average weight by more than ±5%.All the metformin hydrochloride brands used satisfied this specification and therefore passed the uniformity of weight test.
Friability Test
From table 2, all the unexpired brands of metformin tablets used have higher percentage weight loss compared to the expired brands. All the brands except C1 and F1 Complied to the BP 2013, (within the range of not more than 1%). C1 (1.08%), F1 (5.75%), which shows that the increase in percentage weight loss is as a result of degraded products present.
Hardness Test
A 4kgf diametric crushing force is the minimum crushing force for satisfactory metformin tablets, all the expired and unexpired metformin tablets brand used except B1 has low crushing force compared to the same brand. And all the expired brands have lower crushing strength compared to the unexpired brands which shows that the expired tablets are degrading.
Dissolution Profile
According to the British pharmacopoeia 2013, for each of the tablets tested for dissolution, not less than 70% of labelled amount of metformin tablets should be dissolved in 45 min. For the dissolution test, all the unexpired brands of metformin tablets met the 70% drug released at 45 min following (BP 2013) specification, the results revealed that expired brand A1, D1,E1, F1 has lower percentage drug release at 45 min. A1(64%), D1(69.8%), E1(69.7%), F1(69.9%). The unexpired brands has higher percentage of drug released at 45 min compared to the unexpired, showing reduction in dissolution profile for the expired brands.
Assay Content
The content assay for the expired and unexpired brands of metformin hydrochloride was carried out by using Ultraviolet spectrophotometric (UV) method, the percentage content and mg content of the expired brands are lower than the unexpired brands, showing deviation from the unexpired brands of metformin.
CONCLUSION
All of the unexpired brands of metformin met pharmacopoeia requirements. All the unexpired brands complied with the official specification for weight uniformity, friability test, hardness test, dissolution test and assay content, and identification test, however for the expired metformin tablet brands some failed the official specifications as a result of some degraded products. Some of the expired tablets have degraded products.
FUNDING: This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
REFERENCES
- Adegbolagun OA, Olalade OA, Osumah SE. Troical J Pharm Res. 2007, 6:737-45.
- Akinleye MO, Adelaja IA, Odulaja JO. J App Pharm Sci. 2012, 02: p. 41-44.
- Akwasi S, Kwabena O, Noble k, et al., British J pharma Res. 2012, 9: p. 1-14.
- Amidon GL, Lennernäs H, Shah VP, et al., Pharm Res. 1995, 12: p. 413-20.
- Anderson NH, Bauer M, Boussac N, et al., J pharm biomed anal. 1998, 17: p. 811-22.
- Arcot RC, Chan YJ, Choong SH. J applied pharmaceutical sciences. 2011, 01: p. 214-217.
- Awofisayo SO, Awofisayo OA, Eyen N, et al., Dissolution Technology. 2010, 17(2): p. 20-25.
- Bai G, Wang Y, Amenante PM. Int J Pharm. 2011, 403(1): p. 1-14.
- Banakar UV. J pharm sci New York. 1992, 49: p. 347-384.
- Bao W, Srinivasan SR, Berenson GS. The Bogalusa Heart Study. 1996, 93: p. 54-59.
- Blume HH, Schug BS. Eur J Pharma Sci. 1999, 9(2):p. 117-121.
- Cook JA, Bockbrader HN. Dissolution Technology. 2002, 9(2): p. 6-8.
- Fujii A, Yasui-Furukori N, Nakagami T, et al., Drug Description and Development in Therapeutics. 2009, 2: p. 139-44.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref