Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 5
Chemical Composition, Antioxidant and Antibacterial Activity of Thymelaea microphylla Essential Oil from Algeria
Souheila Bounab1, Lograda Takia1, Messaoud Ramdani1*, Pierre Chalard2 and Gilles Figueredo3
1Laboratory of Natural Resource Valorisation, SNV Faculty, Setif 1 University, 19000 Setif, Algeria
2SIGMA Clermont, Campus Des Cezeaux, CS 20265, 63178 Aubière Cedex, France
3LEXVA Analytique, 460 Rue Du Montant, 63110 Beaumont, France
- *Corresponding Author:
- Messaoud Ramdani
Laboratory of Natural Resource Valorisation, SNV Faculty
Setif 1 University
19000 Setif, Algeria
Abstract
The chemical composition of essential oil, isolated from Thymelaea microphylla by hydrodistillation, was analysed by GC and GC/MS. A total 30 compounds representing 99.91% of the oil were identified in Dreaat population. The essential oil of T. microphylla is Characterized by a high rate of Tridecanal (31.24%), Nonanal<n-> (11.43%), Pentadecen 2-one (6Z) (7.93%) and the Citronellol (6.80%). Antioxidant capacity was determined using the method of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. The extracts essential oil showed a good antioxidant activity. Disc diffusion method was used to evaluate the antibacterial activity on five different strains. The oil showed a significant effect against Gram-negative and Gram-positive bacteria.
Keywords
Essential oil, Chemical composition, Antioxidant activity, Antibacterial activity, Thymelaea microphylla, Algeria
Introduction
Most of the natural products reported and listed in the genus Thymelaea, belong to the large group of polyphenolic compounds and more precisely to flavonoids [1-6]. Compounds of the sterol family are reported in Thymelaea microphylla [5] and in Thymelaea hirsuta [1]. T. microphylla, T. hirsuta, Thymelaea lythroides and Thymelaea tartonraira are very rich in coumarines, [1,7,5]. The microphybenzimidazole compound was isolated from T. microphylla [8].
The essential oils of the genus Thymelaea, have been the subject of several phytochemical studies. T. hirsuta oils are characterized by high amounts of sesquiterpenes and monoterpenes [9,10]. The T. microphylla essential oil composition is dominated by the monoterpenes (67.84%) [11], and the study of its ethanolic extracts showed the presence of monoterpenes glycosides [12].
T. hirsuta is known for its powerful antiseptic, hypoglycemic and anti-inflammatory properties and for the treatment of hypertension, such as antimelanogenesis, antidirehyde and purgative and in the treatment of influenza [1,2,5,6,10,13-17]. In M'Sila region, T. hirsuta is recommended by herbalists for the treatment of human diseases (Leishmanicide, dewormer and eczema) [18]. In Algeria anticancer and antiinflammatory effects of T. micrphylla methanol extracts have been tested [5,19,20]. T. lythroides extracts have strong antifungal activity [2].
T. microphylla essential oil showed a high antibacterial activity against Escherchia coli, Staphylococcus aureus, Klebsiella pneumoniae, and a weak inhibitory effect against Pseudomonas aeruginosa [21]. The essential oil of T. hirsuta, shows antioxidant, antimicrobial and antifungal activities [9,16,19]. The cytotoxicity of the oil of this species is demonstrated by Felhi et al. [16].
The objective of this study is to determine the chemical composition of T. microphylla, species endemic to the Sahara, and to evaluate the antibacterial and antioxidant activities of its essential oil.
Materials and Methods
Plant material
T. microphylla Coss. & Dur., commonly called "Methnane Ghazal or Methnane Labiadh", is a plant endemic to North Africa. It is a woody plant with much branched stems whose leaves are small, ovoid, scattered and distant on the branches. Flowers are glomerulate 2-5 in the axils of leaves, yellowish in colour with very short lobes. This plant is found in arid and desert pastures (Figure 1) [22].
T. microphylla is collected from the Dreaat forest (Hammam Dalaa, M’Sila). Aerial parts were collected during the flowering stage in Mai 2017 (Figure 2).
The air dried materials were subjected to hydro-distillation for 3 h using a Clevenger apparatus type. Voucher specimens were deposited in the herbarium of the Department of Biology and Ecology, Setif University, Algeria. The oil obtained was collected and dried over anhydrous sodium sulphate and stored in screw capped glass vials in a refrigerator at 4-5°C prior to analysis. Yield based on dried weight of the samples was calculated.
Essential oil analysis
The essential oils were analysed on a Hewlett-Packard gas chromatograph CPG/FID 7890, coupled to a gaz chromatograph: CPG/MS 7890/5975C, equipped with a Colonn Apolar: DB5 MS: 40 m, 0.18 mm, 0.18 μm, programming from 50°C for 5 min-5°C/min until 300°C. Helium was used as the carrier gas (1.0 ml/min); injection in split mode (1: 30), injector and detector temperature is 280°C with split 1/100. The mass spectrometer worked in EI mode at 70 eV; electron multiplier, 2500 V; ion source temperature, 180°C; MS data were acquired in the scan mode in the m/z range 33-450. The identification of the components was based on comparison of their mass spectra with those of NIST mass spectral library [23,24] and those described by Adams as well as on comparison of their retention indices either with those of authentic compounds or with literature values [25].
Antibacterial activity
The Extract Essential oil was tested against the following bacteria; 2 Gram-negative bacteria: E. coli ATCC 25922; and P. aeruginosa ATCC 27853, and 3 Gram-positive bacteria; B. subtilus ATCC 6633, S. aureus ATCC 25923 and E. faecalis ATCC 51299. The in vitro antibacterial activity of the examined extract was assessed the determination of the activity by the micro dilution method, according to recommendations of the Clinical and Laboratory Standards Institute (NCCLS). The bacterial inoculums was prepared from overnight broth culture in physiological saline (0.8% of NaCl) in order to obtain an optical density ranging from 0.08-01 at 625 nm. Muller-Hinton agar (MH agar) and MH agar supplemented with 5% sheep blood for fastidious bacteria were poured in Petri dishes, solidified and surface dried before inoculation. Sterile discs (6 mm ) were placed on inoculated agars, by test bacteria, filled with 10 l of mother solution and diluted essential oil (1: 1, 1: 2, 1: 4, 1: 8 and 1: 16 v:v of Dimethyl Sulfoxide (DMSO)). DMSO was used as negative control. Bacterial growth inhibition was determined as the diameter of the inhibition zones around the discs. All tests were performed in triplicate. Then, Petri dishes were incubated at 37°C during 18 to 24 h aerobically (Bacteria). After incubation, inhibition zone diameters were measured and documented.
Antioxidant activity
The free radical-scavenging activity of T. microphylla crude extract was measured in terms of hydrogen donating or radical-scavenging ability using the stable radical DPPH. In this assay, the purple chromogen radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) is reduced by antioxidant/reducing compounds to the corresponding pale yellow hydrazine [26]. The scavenging capacity is generally evaluated in organic media by monitoring the absorbance decreases at 517 nm until the absorbance remains constant. 4.0 mM solution of DPPH in methanol was prepared and 1.0 ml of this solution was added to 2.9 ml of extract solution in methanol at different concentrations (37.5 to 300 μl). Thirty minutes later, the absorbance was measured at 517 nm. BHT (Butelated hydroxytoluen) was used as the standard. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. Radical-scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the following formula:
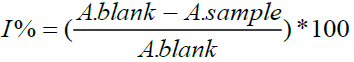
Where (A. blank) was the absorbance of the control (blank, without essential oil) and (A. sample) was the absorbance in the presence of the essential oil. All the tests were performed in triplicate and the graph was plotted with the mean values [27].
Results
Chemical analysis
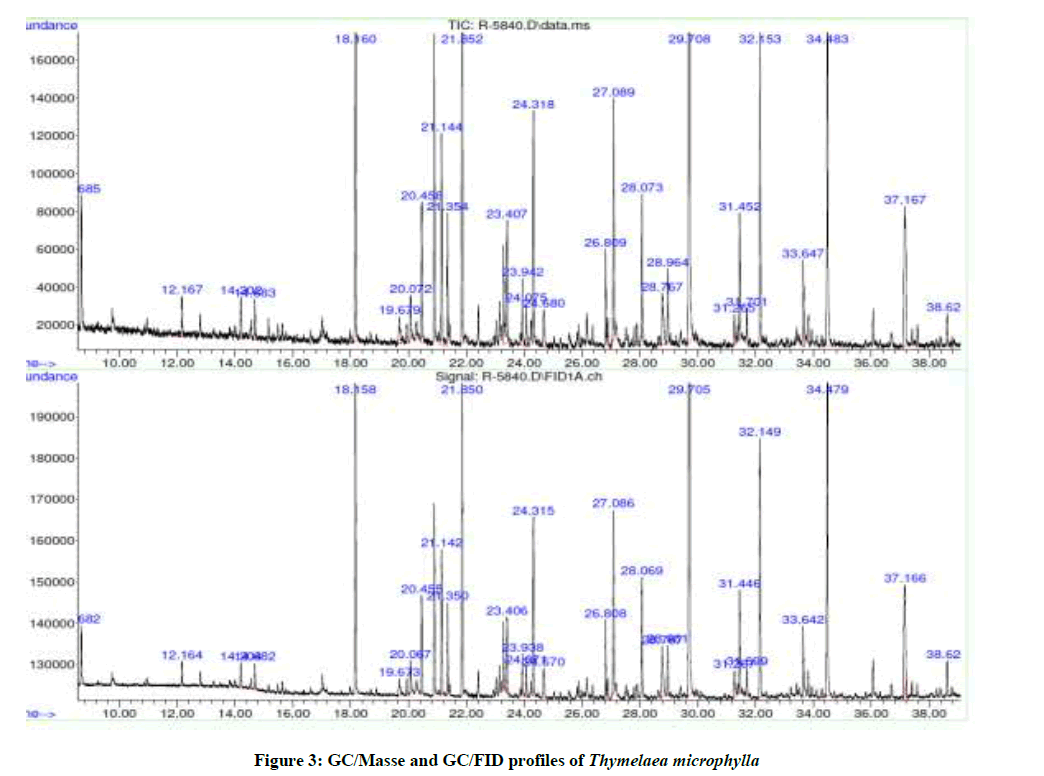
The hydro-distillation of T. microphylla essential oil gave a viscous liquid with pale yellow oil. The average yield of essential oil of the sample is 0.14%. The analysis and identification of the components of the essential oil of this species was performed using the GC-GC/MS (Figure 3). The compound identified in these oils and the abundances are presented in order of their appearance (Table 1).
| Yield % | KI | 0.14 | Yield % | KI | 0.14 |
|---|---|---|---|---|---|
| Number of compound | 30 | Number of compound | 30 | ||
| Total | 99.91 | Total | 99.91 | ||
| Pentanone-4-hydroxy-4-methyl-2 | 838 | 1.76 | Citronellyl acetate | 1322 | 0.89 |
| α-pinene | 935 | 0.55 | Tetradecane (C14) | 1399 | 1.55 |
| Pentyl furan-2 | 989 | 0.62 | Dodecanal | 1410 | 3.27 |
| Octanal | 1002 | 0.58 | Neryl acetone | 1449 | 2.27 |
| Nonanal-n | 1102 | 11.43 | Dodecen 1-ol (2E) | 1476 | 1.41 |
| Camphor | 1151 | 0.56 | β-ionone (E) | 1483 | 1.11 |
| Lavandulol | 1164 | 0.57 | Tridecanal | 1513 | 31.24 |
| Naphthalene | 1191 | 1.94 | Hexenyl benzoate (3Z) | 1577 | 0.54 |
| Dodecane | 1200 | 2.8 | Tridecen 1-al (2E) | 1585 | 1.74 |
| Decanal-n | 1207 | 1.64 | Sesquisabinene hydrate Trans | 1595 | 0.57 |
| Citronellol | 1224 | 6.8 | Tetradecanal | 1614 | 4.43 |
| Lavandulyl acetate tetrahydro | 1273 | 3.5 | Isomenthone 2 (3-oxobutyl) | 1679 | 1.35 |
| Isopulrgyl acetate iso | 1295 | 1.06 | Pentadecen-2-one (6Z) | 1716 | 7.93 |
| Tridecane | 1300 | 0.55 | Caryophyllene 14, 6 hydroxy 4-5 dihydro | 1841 | 3.65 |
| Undecanal | 1309 | 3.09 | Farnesyl acetone (5E, 9Z) | 1912 | 0.51 |
Table 1: Chemical composition of Thymelaea microphylla essential oil
This analysis led to the identification of 30 components representing 99.93% of the total oil of T. microphylla. According to our results the chemical composition of the species, T. microphylla is dominated by the presence of major compounds, the Tridecanal (31.24%), Nonanal<n-> (11.43%), Pentadecen 2-one (6Z) (7.93%) and the Citronellol (6.80%). As well as other component with lower percentages, Tetradecanal (4.43%), Caryophyllene 14, 6-hydroxy 4-5 dihydro (3.65%), Lavandulyl acetate tetrahydro (3.50%), Dodecanal (3.27%), Undecanal (3.09%) and the presence in trace of other compounds.
Antibacterial activity
The results of the experiments assessing the bacteriostatic effects of T. microphylla essential oil of the study on Gram-negative and Gram-positive bacteria are presented in (Table 2).
| Antibiotic* | Dilution | ||||||
|---|---|---|---|---|---|---|---|
| Bacteria | Gen | Amo | 1/1 | 1/2 | 1/4 | 1/8 | 1/16 |
| Bacillus subtillus ATCC 6633 | 26 | - | 29 | 19.5 | 18.5 | 14 | 11.5 |
| Echerichia coli ATCC 25922 | 26 | - | 26.5 | 26 | 16.5 | 15.5 | 13.5 |
| Enterococcus faecalis ATCC 51299 | - | 26 | 18.5 | 17.5 | 15.5 | 15 | 14.5 |
| Pseudomonas aureiginosa ATCC 27853 | 28 | - | - | - | - | - | - |
| Staphyllococcus aureus ATCC 25923 | 30 | - | - | - | - | - | - |
*Gen= Gentamicine; Amo= Amoxicilline
Table 2: Antibacterial activity of Thymelaea microphylla essential oil
Effectively, the essential oil from T. microphylla leaves were demonstrated antibacterial activity against the gram negative clinical pathogens bacteria tested, B. subtillus, E. coli and E. faecalis, bat no activity against the bacteria P. aureiginosa and S. aureus.
Antioxidant activity
The antiradical activity of T. microphylla essential oil is evaluated by their inhibitory capacity of a methanolic solution of DPPH; it’s measured by spectrophotometer at 517 nm. The standard used was BHT (Butylated hydroxytoluen) (Table 3). The change in the colour of the solution containing T. microphylla essential oil proves that the oil has antioxidant activity.
| Concentrations | 300 μl (1/1) | 150 μl (1/2) | 75 μl (1/4) | 37.5 μl (1/8) |
|---|---|---|---|---|
| Absorbance of EO (nm) | 0.057 | 0.119 | 0.317 | 0.515 |
| Absorbance de BHT (nm) | 0.068 | 0.14 | 0.33 | 0.64 |
| Inhibition (%) of EO | 91.53 | 82.31 | 52.89 | 23.47 |
| Inhibition (%) of BHT | 89.89 | 79.19 | 50.96 | 4.9 |
Table 3: Antioxidant activity of essential oil of Thymelaea microphylla
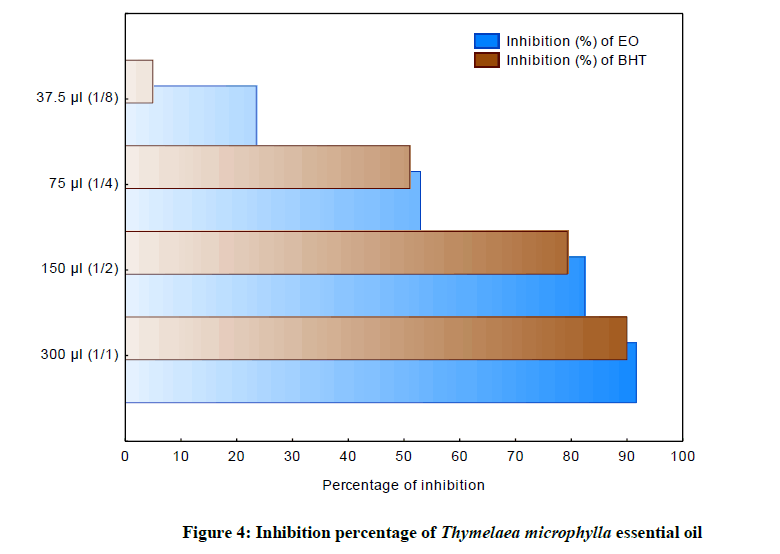
The inhibition percentages of different concentrations of T. microphylla essential oil as well as the BHT standard fluctuate between 4.90% and 89.89% for BHT, 23.47% and 91.53% for T. microphylla oil (Figure 4). It should be noted that there is a certain similarity in the percentages of inhibition, for the oil and for the BHT standard, except for the 1/8 dilution.
The highest inhibition value is 91.53% for oil and 89.89% for BHT, indicating a possibility that T. microphylla oil contains a greater amount of free radical accepting compounds, as well as that the greatest antioxidant potential. The percentage of inhibition of the free radical increases with the increase of the concentration, either for the BHT or for the essential oil. IC50 is inversely related to the antioxidant capacity of a compound because it expresses the amount of antioxidant required to decrease the free radical concentration by 50%.
Discussion
Many works have shown a great heterogeneity in the chemical composition of the genus Thymelaea. In Tunisia, T. hirsuta is characterized by heptane, germacrene-D, γ-eudesmol and citronellyl formate [9,28]. The T. hirsuta essential oil, cultivated in Tunisia, is dominated by hexadecanoic acid, 4, 8-and 13- dimethylhecosane methylhexacosane [10].
Chemical analysis of T. microphylla essential oils allowed us to identify tridecanal (31.24%), nonanal <n-> (11.43%), pentadecen (7.93%) and citronelloll (6.80%) as major components. The chemical results differ from those cited in the literature. T. microphylla samples from the Ourgla region (Algeria) show the predominance of D-menthone, 2-undeconeone, pulegone and perillal [11]. This difference in composition is probably due to various conditions including the environment, geographical origin, harvest period, temperature and drying time.
T. hirsuta essential oil shows significant antioxidant activity [17,29]. This oil reduces the formation of DPPH radicals in a dose-dependent manner [9]. The investigation of the antioxidant activity of the essential oil of T. micropylla has shown that this species has antioxidant activity. The various studies of aqueous and methanolic extracts of T. microphylla prove the presence of antioxidant activity [3,6,12,20,21,30].
Species of the genus Thymelaea exhibit important biological activities. The essential oil of T. hirsuta has moderate antimicrobial activity against all microorganisms tested [16,19]. T. lythroides has a strong antifungal activity [2] whereas the methanolic extracts of T. micropylla showed no bacteriological effect [20].
Our investigation shows a high activity of T. micriphylla essential oil on B. subtillus, E. coli, E. faecalis bacteria and no effect against P. aureiginosa and S. aureus.
The studies of Noman et al. [31] showed high antibacterial activity of T. microphylla essential oil against E. coli and S. aureus, K. pneumoniae and a weak inhibitory effect against P. aeruginosa. The absence of antibacterial activity can be explained by the developed resistance of some strains that react differently to several essential oils; this is the case of P. aeruginosa [32,33].
Conclusion
This study aims to identify the chemical composition of T. microphylla essential oils and the evaluation of their antioxidant and antibacterial activities. The analysis of the chemical composition of the essential oil by GC/MS has allowed the identification of 30 compounds. The Tridecanal is the major component of the chemical composition, although the study has identified the other natural products as minor components. The results exhibited antioxidant activity, which are stronger than the positive control BHT. An additional characteristic of T. microphylla essential oil was its prominent antibacterial activity against B. subtillus, E. coli, E. faecalis and no effect against P. aureiginosa and S. aureus. The Essential oil may be a promising alternative treatment of localized infections even with severe hospital acquired strains. Further investigations must be made to determine the active constituent(s) for their application in medical research.
Acknowledgement
The work was supported by Algerian MESRS and Chemical Laboratory of carbohydrates Heterocyclic of Clermont Ferrant, France.
References
- N. Dohou, K. Yamni, S. Tahrouch, L.M. Idrissi Hassani, A. Badoc, N. Gmira, Bull. Soc. Pharm. Bordeaux., 2003, 142, 61-78.
- N. Dohou, K. YAMNI, A. Badoc, A. Douira, Bull. Soc. Pharm. Bordeaux., 2004, 143, 31-38.
- N. Benhammou F. Atik Bekkara, JM. Coustard, Adv. Food Sci., 2009, 31(4), 194-201.
- H. Ghanem, H. Haba, L. Marcourt, M. Benkhaled, J.L. Wolfender, Nat. Prod. Res., 2004, 28(20), 1732-1738.
- T. Mekhelfi, PhD thesis in Pharmaceutical Chemistry, University of Constantine, Algeria, 2016.
- K. Kerbab, PhD thesis in Pharmaceutical Chemistry, University of Constantine, Algeria, 2017.
- T. Mekhelfi, K. Kerbab, G. Guella, L. Zaiter, S. Benayache, F. Benayache, Der Pharmacia Lettre., 2014, 6(1), 152-156.
- L. Noman, F. Oke-Altuntas, A. Zellagui, A.S. Yaglioglu, I. Demirtas, S.M. Cardoso, S. Akkal, N. Gherraf, S. Rouati, Natural. Product. Research. Formerly. Natural. Product. Letters., 2017, 31(17), 2032-2041.
- A. Kadri Z. Zarai, I. Ben Chobba, N. Gharsallah, M. Damak, A. Békir, African J. Biotechnology., 2011, 10(15), 2930-2935.
- M. Yahyaoui, J.Bouajila, S. Camy, J.S. Condoret, M. Abderabba, International Symposium on Essential oils natural volatiles & Essential oils, 2014, Istambul, Turky.
- L. Noamane, A. Zellagui, K. Mesbah, N. Gherraf, M. Lahouel, S. Rhouati, Der Pharmacia Lettre., 2010, 2(5), 428-431.
- K. Kerbab, T. Mekhelfi, L. Zaiter, S. Benayache, F. Benayache, P. Picerno, T. Mencherini, F. Sansone, R.P. Aquino, L. Rastrelli, Natural Product Research, Formerly Natural Product Letters., 2014, 29(7), 671-675.
- A. Ziyyat, A. Legssyer, H. Mekhfi, A. Dassouli, M. Serhrouchni, W. Benjelloun, J. Ethnopharmacol., 1997, 58, 45-54.
- N. Dohou, K. Yamni, N. Gmira, L.M. Idrissi Hassani, Acta Botanica Malacitana., 2004, 29, 233-239.
- M. Bnouham, W. Benalia, S. Bellahcen, Z. Hakkou, A. Ziyyat, H. Mekhfi, M. Aziz, A. Legssyer, J. Diabetes., 2012, 4, 307–313.
- S. Felhi, M. Chaaibia, S. Bakari, R. Ben Mansour, A. Békir, A. Gharsallah, A. Adel Kadri, Pak. J. Pharm. Sci., 2017, 30(1), 087-091.
- M. Yahyaoui, N. Ghazouani, I. Ines Sifaoui, M. Abderrabba, Biosci., Biotech. Res. Asia., 2017, 14(3), 997-1007.
- A. Boudjelal, C. Henchiri, M. Sari, D. Sarri, H. Hendel, A. Benkhaled, G. Ruberto, J. Ethnopharmacol., 2013, 148(2), 395-402.
- S. Dahamna, K. Dehimi, M. Merghem, M. Djarmouni, D. Bouamra, D. Harzallah, S. Khennouf, International Journal of Phytocosmetics and Natural Ingredients., 2015, 2, 15-18.
- K. Dehimi, A. Speciale, A. Saija, S. Dahamna, R. Raciti, F. Cimino, M. Cristani, Phcog. Mag., 2016, 12(47), 203-210.
- L. Noman, A. Zellagui, Y. Hallis, S. Yaglioglu, I. Demirtas, N. Gherraf, S. Rhouati, Der Pharmacia Lettre., 2015, 7(1), 118-121.
- P. Quézel, S. Santa, Ed. CNRS, Paris, France, 1963.
- Y. Masada, J. Wiley & Sons, Inc. New York, 1976.
- NIST, Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library, vers. 2.0. fiveash data, USA, 2002.
- RP. Adams, Allured Publishing Corporation Carol Stream, Illinois USA, 2007.
- . F. Epifano, S. Genovese, L. Menghini, M. Curini, Phytochemisty., 2007, 68, 939 - 953.
- B. Archana, N. Dasgupta, B. De, B. Food Chem., 2005, 90, 727-733.
- I. Ben Chobba, S. Felhi, M. Chaaibia, R. Ben Mansour, A. Békir, N. Drira, N. Gharsallah, A. Kadri, The Third International Symposium on the Biology of Rare and Endemic Plant Species (BIORARE-2014) April 19-23, Antalya, Turkey, 2014.
- O.N. Amari, M. Bouzouina, A. Berkan, B. Lotmani, Asian. Pac. J. Trop. Dis., 2014, 4(2), 104-0109.
- N. Benhammou, PhD Thesis in Biology, Option: Natural Substances, Biological Activities and Synthesis. University Aboubakr Belkaïd Tlemcen (Algeria), 2012.
- L. Noman, A. Zellagui, A.S. Yaglioglu, I. Demirtas, S. Rhouati, Res. J. Pharm., Biol. Chem. Sci., 2015, 6(2), 671-676.
- C.M. Mann, S.D. Cox, J.L. Markham, Lett. Appl. Microbiol., 2000, 30, 294-297.
- K.A. Hammer, C.F. Carson, T.V. Riley, J. Appl. Microbiol.,1999, 86, 985-990.