Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Calotropis gigantea Leaves Ark Used as Corrosion Inhibitor for Aluminum in Hydrochloric Acid
Desai PS*
Department of Chemistry, Arts, Science and Commerce College, Kamrej Char Rasta, Surat-394185, Gujarat, India
- Corresponding Author:
- Desai PS
Department of Chemistry Arts, Science and Commerce College
Kamrej Char Rasta, Surat-394185, Gujarat, India
Abstract
Inhibition of aluminum corrosion in 0.4-0.6 M hydrochloric acid solution in the presence and absence of using Calotropis gigantea (Aankado) leaves ark’s has been studied employing gravimetric and electrochemical techniques. The results showed that the C. gigantea barks could serve as an effective inhibitor for the corrosion of aluminum in HCl solutions. Inhibition was found to increase with increasing concentration of the extract but decreases with increasing acid concentration. The adsorption study support that Freundlich isotherm best fit to the metal surface interaction with the C. gigantea leaves ark’s with the finest exposure time for the adsorb to the metal surface at all concentrations. Polarization study reveals that the inhibitors functions as a mixed type of inhibitor.
Keywords
Adsorption, Aluminum, Calotropis gigantea (Aankado), Corrosion, Freundlich isotherm, Hydrochloric acid
Introduction
Aluminum is non-magnetic and non-combustible, properties invaluable in advanced industries such as electronics or in offshore structures. Aluminum is non-toxic and impervious, qualities that have established its use in the food and packaging industries since the earliest times. A key property is low density. Aluminum is only one-third the weight of steel. Aluminum tanks in aircraft fuel systems are subject to corrosion resulting from the growth of microbiological constituents in kerosene fuel [1-4].
Aluminum and most of its alloys are highly resistant to most forms of corrosion. The metal’s natural coating of aluminum oxide provides a highly effective barrier to the ravages of air, temperature, moisture and chemical attack. Therefore, aluminum alloy is known to exhibit passive behavior in aqueous solutions. This is may be due to the formation as protective, tightly adherent and at times invisible oxide film on the metal surface. The film is generally stable in solutions of pH 4.5-8.5 [5]. However, due to the solubility as the film in strong acid and strong alkaline media, the metal exhibits high rate of corrosion. However, to prevent the attack of acid, it is very important to add some corrosion inhibitor to decrease the corrosion rate of aluminum in such solution. A huge number of organic compounds [6-11] are renowned to be applicable as corrosion inhibitors for aluminum in acidic solution. Such organic molecules characteristically contain nitrogen, oxygen or sulfur in a conjugated system. Corrosion inhibitions take place via adsorption of the molecules on the metal surface through known adsorption isotherms.
The investigation of natural products of plant origin as cheap green corrosion inhibitors is a necessary pasture of study. In addition to being environmental friendly and ecologically acceptable plant products are cheap, easily available and renewable source of materials [12]. The extract from their leaves, stem, roots and fruits comprise of mixtures of organic compounds like pigments, alkaloids, amino acids etc., and are known to effective inhibitors for metal corrodent system. The use of natural products of plant origin as inhibitors been reported by some workers [13-25] but their use for controlling the corrosion of aluminum in acidic solution has not been investigated till now.
Calotropis is a plant. Many people use the bark and root bark for medicine purpose. It is widely used for digestive disorders including diarrhea, constipation and stomach ulcers for painful conditions including toothache, cramps and joint pain. Some people use Calotropis for syphilis, boils, inflammation epilepsy, hysteria, fever, muscular spasm, warts leprosy, gout snakebites and cancer. It can also be used for inhalation therapy; smoke from bark is inhaled for coughs, asthma and to cause sweating. The current work is experiment to find a cheap and environmentally safe inhibitor for aluminum corrosion in the HCl solution, where the aqueous ark of Aankado plant leaf is tested. This plant belongs to the family Apocynaceae and has the scientific name Calotropis gigantea. Weight loss measurements and potentiostatic polarization techniques are used, in the present work, to compute the inhibition efficiency of the C. gigantea extract. Furthermore, the ability of the extract to provide a protection against pitting corrosion of aluminum in chloride ion containing solutions was studied using potentiodynamic polarization
Materials and Methods
The chemical composition of aluminum alloy contain Si-0.49%, Fe-0.68%, Cu-0.082%, Mn-0.16%, Mg-0.37% and the remainder being Al- 98.02% were used for the corrosion study. The coupons were prepared from the rectangular aluminum sheet of thickness 0.12 cm. The plate was mechanically press-cut into coupon of dimensions 5.0 × 2.0 cm. These coupons were used “as cut” condition, without further polishing and degreased in absolute ethanol, dried in acetone, weighed and stored in a moisture-free desiccators. All chemicals and reagents used were of analytical grade and used as source without further purification. The aggressive media was 0.4-0.6 M HCl solution.
Inhibiting stock solutions of the plant arks were prepared by the C. gigantea leaves were dried grind to powder form and boiling with distilled water to making extract. Inhibitor C. gigantea (Aankado) leaves ark’s was used in the concentration range 0.25-1.25%.
Weight loss experiments
Corrosion damage is most commonly assessed by weight loss method, rectangular specimen having area of 0.2259 cm2 with small hole of about 5 mm diameter near the upper edge of the specimen for suspension have been used. After cleaning and weighting each specimen was suspended to same depth of 1.5 cm below the surface of the aqueous liquid. The volume of the corrosive media for, all experiment was kept 230 ml. Only one specimen was suspended in each glass beaker of 250 ml capacity. Experiments were conducted at room temperature (303 ± 1 K). For satisfactory assessment of corrosion, it is essential to remove corrosion products from the specimen at the same time. The cleaned and dried specimens were weighed before immersion in the respective test solutions of 0.4, 0.5 and 0.6 M HCl for 24 h using CAH 123 electronic weighing balance with the accuracy of ± 0.001. Tests were conducted with different concentrations of inhibitor.
After exposure period, test specimens were removed from the corrosive environment and were cleaned after the test with chromic-phosphate mixture solution. After cleaning the test specimens were washed with distilled water followed by acetone and then dried with air dryer and finally reweighed to determine corrosion loss. Triplicate experiments were performed in each case and the mean values reported.
Polarization
When electrochemical corrosion occurs, the current that flows between anode and cathode causes a change in the electrode potential, this change is termed as polarization. Electrochemical experiments were carried out in a conventional three-electrode glass cell of capacity 230 ml, using a Gamry Potentiostat/Galvanostat electrochemical computer unit. For the polarization study, metal specimens of rectangular design having an area of 1 mm2 were exposed to corrosive solutions. Aluminum was used as a working electrode, SCE was used as reference electrode and the auxiliary graphite electrode was placed in corrosive media through which external current was supplied automatically from the computerized polarization instrument. The change in potential was measured by potentiostat/galvanostat on the potentiostat mode with 5 mV/sec scan rate. Polarization has been taken with and without inhibitors in 0.4 M HCl. The curves show polarization of both the anodes and cathodes.
Result and Discussion
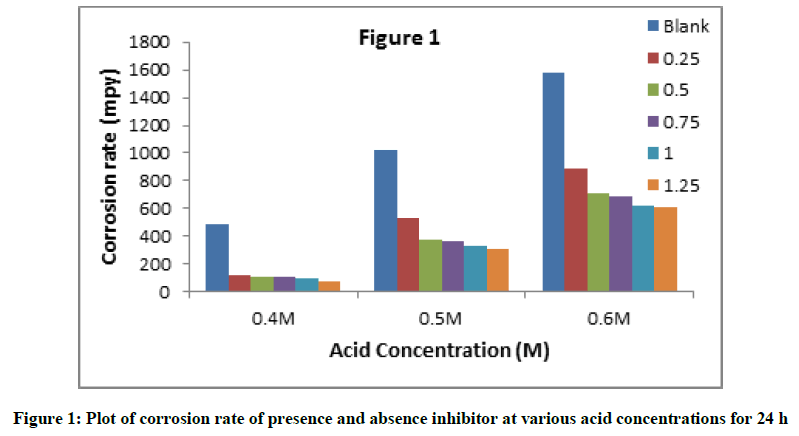
The results obtained for the variation of weight loss with exposure time and for their determined corrosion rates after 24 h for the aluminum test coupon immersed in the 0.4-0.6 M HCl at 30°C are presented in Figure 1 and Table 1. The corrosion rate was calculated from the weight loss data with help of Equation 1. Where, ‘W’ is the weight loss of aluminum in grams, ‘A’ is the surface area of specimen in inches square, ‘D’ is the density of aluminum and ‘t’ is the time in hours.
| Inhibitor concentration (%) | Acid concentration | ||
|---|---|---|---|
| 0.4 M | 0.5 M | 0.6 M | |
| θ | θ | θ | |
| 0.25 | 0.7505 | 0.4827 | 0.4398 |
| 0.50 | 0.7793 | 0.6305 | 0.5516 |
| 0.75 | 0.7843 | 0.6490 | 0.5694 |
| 1.00 | 0.7990 | 0.6767 | 0.6052 |
| 1.25 | 0.8431 | 0.7021 | 0.6171 |
Table 1: Surface coverage area of aluminum in hydrochloric acid environment containing Calotropis gigantea inhibitors for 24 h at 303 K
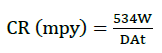 (1)
(1)
The inhibition efficiency (η%) and degree of surface coverage (θ) at each concentration of ark of C. gigantea extract were calculated by comparing the corrosion rate in absence (CRblank) and presence of inhibitor (CRinh) using the relationships:
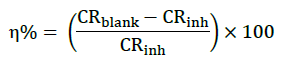 (2)
(2)
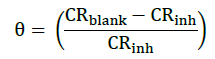 (3)
(3)
The results were reveal that corrosion rates values of aluminum alloy decreased with the increasing concentration of C. gigantean extract in 0.4- 0.5 M HCl in all the concentration ranging from 0.25-1.25%. The corrosion rate increases when the concentration of acid solutions was increase.
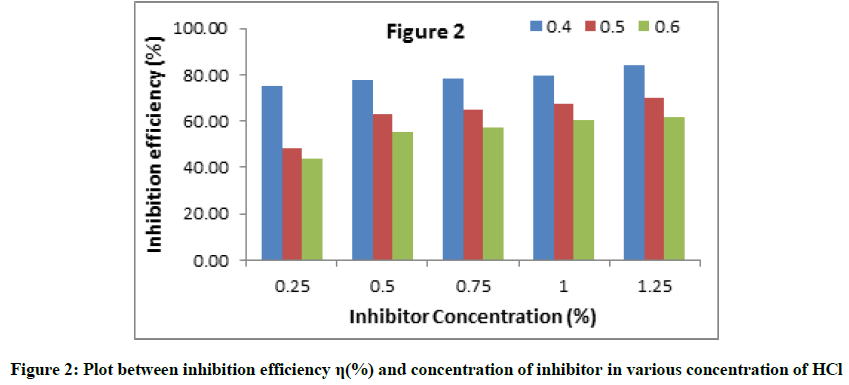
The difference of inhibition efficiency with concentration of C. gigantean leaf extract in 0.4 to 0.6 M HCl solution by weight loss measurements is depicted in Figure 2. It is observed that C. gigantean leaf extract was more effective in inhibiting the corrosion of aluminum in 0.4 M HCl solution at lower concentrations than at higher concentrations. Furthermore, increase in inhibition efficiency with increase in inhibitor concentration indicates a strong interaction between the aluminum metal surface and the inhibitor. The inhibition efficiency increases with increasing inhibitor concentration, reaching the best value (84.31%) at 0.25% concentration of leaves extract.
Numbers of adsorption isotherms were applied but the best fit for the adsorption for C. gigantea is Freundlich isotherm. The relationship between degree of surface coverage (θ) and inhibitors concentration (C) in g/l can be represented by the following Freundlich adsorption isotherm [26]:
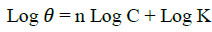 (4)
(4)
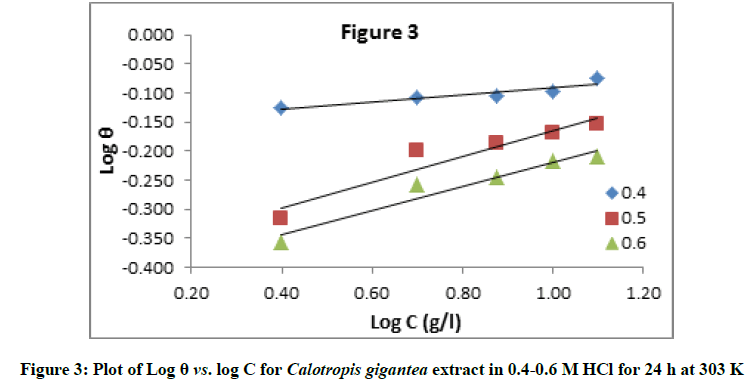
Where, ‘K’ is the equilibrium constant for adsorption and the value of ‘n’ lies in range 0 to 1. Figure 3 shows the plots of Log(θ) versus Log(C) to be linear, with intercept LogK, which indicates that the experimental data fit with the Freundlich adsorption isotherm, showing that the adsorption of extract of Calotropis gigantea on the surface of the aluminum obeys Freundlich’s adsorption isotherm. The values of K were calculated from the intercept of the graph and presented in Table 2. K is related to the standard free energy of adsorption (ΔG0ads) and are calculated in Table 2, using following equation [27,28]:
| Acid concentration | |||
|---|---|---|---|
| 0.4 M | 0.5 M | 0.6 M | |
| Correlation coefficient | 0.838 | 0.912 | 0.941 |
| Intercept | 0.061 | 0.223 | 0.206 |
| K | 7.06 × 10-1 | 4.10 × 10-1 | 3.76 × 10-1 |
| ∆G0ads (Kj/mol-1) | -9.56 | -8.13 | -7.90 |
Table 2: Some parameters of the linear regression of Freundlich adsorption isotherm for aluminum corrosion in 0.4-0.6 M HCl solution containing Calotropis gigantea leaf extract
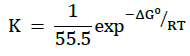 (5)
(5)
Where, R=0.008314 kJ.mol-1’ is the universal gas constant, 55.5 represent the molar concentration of water in the corrodent solution while ‘T’ is the absolute temperature in Kelvin.
From the Table 2, ΔG0ads values are negative in at all cases and lie in the range of -9.56 to -7.90 Kj/mol-1. It is clearly observed from the Table 2, that the acid concentration increases the values of ΔG0ads are decreasing in order (less negative), revealed that the most efficient inhibitor shows more negative ΔG0ads value, tied with a decrease in the inhibition efficiency with increase in acid concentration reveals that physical adsorption. This suggests that they be strongly adsorbed on the metal surface. Generally, values of ΔG0ads less negative than -20 kJ/mol-1 indicate physical adsorption while those more negative than -40 kJ mol-1 indicate chemical adsorption [29,30]. The values ΔG0ads obtained in this experiment being less negative than -20 kJ mol-1 also support physisorption process.
The results clearly showed that the inhibition mechanism involves blocking of the aluminum surface by inhibitor molecules via adsorption. Further clarification of adsorption mechanism from the experimental data the predominant adsorption mode will be dependent on factors such as the extract composition, chemical changes to the extract and the nature of the surface charge on metal. A negative surface charge will favour the adsorption of cations whereas anion adsorption is favored by a positive surface charge. The ability of Cl- ions in hydrochloric acid to be strongly adsorbed on the metal surface and hence facilitate physical adsorption of inhibitor cations is an important consideration [31]. The linear plot with high correlation coefficient (see Table 2) of about unity clearly indicates that the surface adsorption process of Calotropis gigantea extract on the aluminum alloy surface obey the Freundlich adsorption isotherm. It revealed that physical adsorption occurred.
Polarization
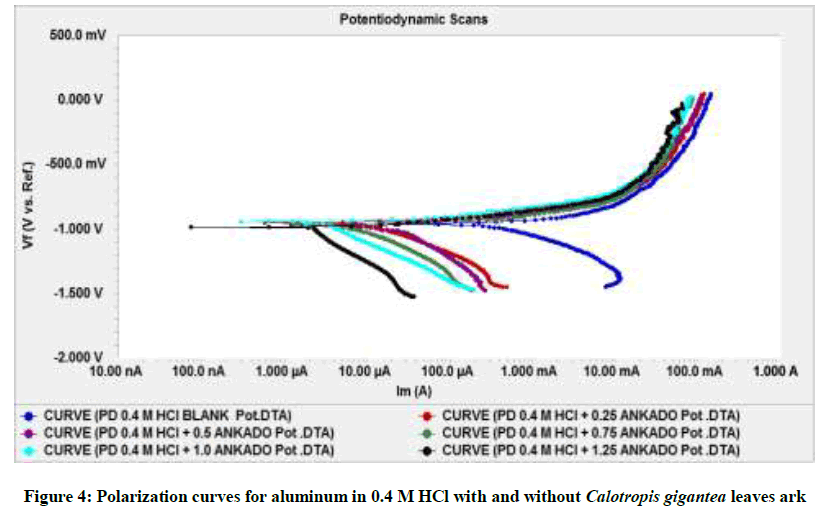
The Tafel polarization graphs of aluminum in HCl solution, in the absence and presence of different concentration of C. gigantean leaves extract, are presented in Figure 4 and listed in Table 3. The values of corrosion current densities in the presence and absence of inhibitor were obtained from the graph while percentage efficiency (η%) was calculated using the following Equation 6.
| Inhibitor concentration (%) | Ecorr mV | icorr (μA/cm2) | Tafel slope (V/decade) | Inhibition efficiency (%) | ||
|---|---|---|---|---|---|---|
| Anodic+βa | Cathodic-βc | Polarization method (icorr) | Weight loss method | |||
| Blank | -958 | 203.0 | 6.87E-02 | 2.53E-01 | - | - |
| 0.25 | -953 | 24.2 | 6.18E-02 | 2.99E-01 | 88.09 | 75.05 |
| 0.50 | -970 | 14.2 | 4.67E-02 | 2.31E-01 | 93.00 | 77.93 |
| 0.75 | -964 | 6.06 | 3.89E-02 | 2.69E-01 | 97.01 | 78.43 |
| 1.00 | -927 | 4.5 | 4.24E-02 | 7.21E-02 | 97.78 | 79.90 |
| 1.25 | -986 | 2.76 | 5.59E-02 | 5.59E-02 | 98.64 | 84.31 |
Table 3: Polarization data and inhibition efficiency of inhibitors for aluminum in 0.4 M HCl
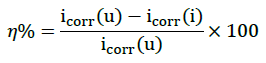 (6)
(6)
Where, icorr(u) and icorr(i) represents the corrosion current for uninhibited and inhibited system respectively while η% represents inhibition efficiency in percentage.
The maximum inhibition efficiency (98%) was obtained at concentration of 1.25%. Addition of the C. gigantea leaf extract to acid solution affected both the cathodic and anodic parts of the curves. C. gigantea extract, influenced both the anodic dissolution of aluminum and the generated hydrogen gas at the cathode indicating that the extract behaved as mixed-type inhibitor. The decrease in the observed limiting current with increasing C. gigantea concentration indicated that the anodic process is controlled by diffusion. From the polarization figure it was noted that the curves were shifted towards the lower current density region and βa and βc values did not showed any significant change. The inhibition values in the Table 3 reflected that the C. gigantea leaf extract acted as very effective corrosion inhibitors for aluminum in acid solution and its capacity of inhibition increased with increase of concentration.
Aluminum alloy dissolves in acid solutions with the evolution hydrogen gas. The reaction-taking place at the micro electrodes of the corrosion cell being represented as under:
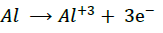 (7) (Anode)
(7) (Anode)
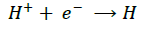 (8) (Cathode)
(8) (Cathode)
Followed by the reaction:
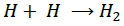 (9)
(9)
The following secondary reaction can also take place in acid solutions [32].
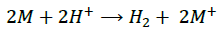 (10) (Anode)
(10) (Anode)
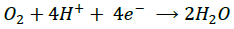 (11) (Cathode)
(11) (Cathode)
Therefore, only if the hydrogen evolution type of attack is predominate and no other factors influence the corrosion process, corroded by the strong acid should be maximized. The inhibitory mechanism is a separation process involving (i) The inhibitor is adsorbed on the surface of the metal forming a compact protective thin layer and (ii) The inhibitor forms a precipitate on the surface of the metal, acting on the aggressive media to form protective precipitates or remove the aggressive agents [33].
The results clearly proved that the inhibition mechanism involves blocking of the aluminum surface by inhibitor molecules via adsorption. In general, the occurrence of adsorption is influenced by the nature of metal and chemical constitution of particular inhibitors which exactly used. C. gigantea leaves extract contain significantly higher concentrations of alkaloids, fatty acids and N and O containing organic molecules. The major chemical constituents of C. gigantea are laurane, saccharose, B-amyrin, A and B calotropeols, holarrhetine, cyanidin-3-rhamnoglucoside, taraxsterol isovalerate, giganteol, calotroposide, calactin, calotoxin, calotropins DI and DII and gigantin [34]. The inhibitive effect is attributed due to the present of organic compounds in the extract. The adsorption of these organic molecules occurred due to the formation of a links between aluminum atoms, involving the displacement of water molecules from metal surface, and the lone pairs present on N- and O- atoms of the heterocyclic rings. Finally it can be concluded that, the Calotropis gigantea leaves extract is effective inhibitor for aluminum corrosion in hydrochloric acid solution.
Conclusion
The extracts from the leaves of Calotropis gigantea found to be effective green inhibitor of aluminum alloy in 0.4 M hydrochloric acid solution. At all concentration of acid, as the inhibitor concentration increases inhibition efficiency increases and corrosion rates decreases. The corrosion process was inhibited by adsorption of the extract of organic molecules on the aluminum surface according Freundlich isotherm. The inhibition efficiency from Tafel plots agrees well the values obtained from weight loss data.
Acknowledgement
The author is thankful to the Gujarat Council on Science and Technology, Department of Science and Technology, Gandhinagar, Gujarat for providing a financial support and also thanks full to Department of Chemistry, Arts, Science and Commerce College, Kholwad, Surat, Gujarat, India for providing laboratory facilities.
References
- J. Elphick, In Microbial Aspects of Metallurgy, edited by J.D.A. Miller, American Elsevier, New York, 1970, 157-172.
- H. Hedrick, Mater. Prol., 1970, 9(1), 27.
- PB. Park, In Microbial Aspects of the Deterioration of Materials, edited by H. Gilbert and D. Lovelock, Academic Press. London, 1975, 105-126.
- R. Sahire, M. de Mele, M. Videla, Corrosion., 1963, 39, 26.
- W.W. Binger, Corrosion Resistance of Metal and Alloy, edited by F.L. Laque, H.R. Copson, Reinhold Publishing Corporation, New York, 1963, 183.
- P.S. Desai, Eur. J. Biomed. Pharm. Sci., 2015, 2(1), 657-668.
- P.S. Desai, S.M. Kapopara, Indian J. Chem. Technol., 2009, 16(6), 485-491.
- P.S. Desai, R.T. Vashi, J. Indian Chem. Soc., 2009, 26(5), 547-550.
- R.T. Vashi, P.S. Desai, Bull. Electrochem., 2007, 23, 87-93.
- P.S. Desai, S.M. Kapopara, Indian J. Chem. Technol., 2014, 21(2), 139-145.
- P.S. Desai, R.T. Vashi, Anti-Corrosion Methods and Materials., 2011, 58(2), 70-75.
- G.O. Avwiri, F.O. Igho, Mater. Lett., 2003, 57(22-23), 3705-3711.
- P.S Desai, GE-Int. J. Eng. Res., 2015a, 3(1), 8-23.
- P.S. Desai, Eur. J. Pharm. Med. Res.,2015b, 2(1), 470-485.
- E.U. Ugwu, O.E. Okore, T.O. Olagbemiro, I.Y. Chindo, J. Chem. Soc. Nigeria., 1997, 22(1), 112-8.
- M. Bouklah, B. Hammouti, Portugaliae Electrochimica Acta.,2006,24, 457.
- E.E. Oguzie, Pigm. Resin Tech., 2005, 34(6), 321.
- P.C. Okafor, U.J. Ekpe, E.E. Ebenso, E.M. Umoren, K.E. Leizou, Bull. Electrochem.,2005,21(8), 347.
- E.E. Oguzie, E.E. Ebenso, Pigm. Resin Tech., 2006, 35(1), 30.
- A.Y. El-Etre, Z. El-Tantawy, Port. Electrochim. Acta.,2006, 24, 347.
- E.E. Oguzie, Pigm. Resin Technol., 2006, 35(6), 334-340.
- A. Singh, E.E. Ebenso, M.A. Quraishi, Int. J. Electrochem. Sci., 2012, 7, 3409.
- M.A. Quraishi, A. Singh, V.K. Singh, D.K. Yadav, A.K. Singh, Mater. Chem. Phys.,2010, 122, 114.
- A. Singh, I. Ahamad, V.K. Singh, M.A. Quraishi, J. Solid State Electrochem., 2011, 15, 1087.
- P.S. Desai, Int. J. Curr. Microbiol. App. Sci.,2015c, 4(1), 437-447.
- A.M. Al-Bonayan, IJRRAS., 2015, 22(2), 49-64.
- A.S. Fouda, A.Y. El-Khateeb, M. Ibrahim, M. Fakih, Nature Sci., 2015, 13(2), 71-82.
- B.I. Ita, O.U. Abakedi, V.N. Osabor, Glo. Adv. Res. Eng. Technol. Innov., 2013, 2(3), 84-89.
- E.I. Ating, S.A. Umoren, I.I. Udousoro, E.E. Ebenso, A.P. Udoh, Green Chem. Lett. Rev., 2010, 3(2), 61-68.
- E.E. Ebenso, Bull. Electrochem.,2003, 19(5), 209-216.
- L.A. Nnanna, B.N. Onwuagba, I.M. Mejeha, K.B. Okeoma, African J. Pure Appl. Chem., 2010, 4(1), 011-016.
- H.P. Godard, W.B. Jepson, M.R. Bothwell, R.L. Kane, The Corrosion of Light Metals, John Wiley and Sons Inc., New York, 1967, 52.
- A.R. El-Sayed, H.S. Mohran, H.M. Abd El-Lateef, Corros. Sci.,2010, 52, 1076.
- http://easyayurveda.com/2013/12/12/calotropis-gigantea-arka-uses-dose-side-effects-ayurveda-details/