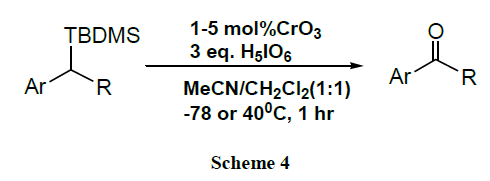Review Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
Applications of Chromium(VI) Complexes as Oxidants in Organic Synthesis: A Brief Review
Sangita D Katre*
Department of Chemistry, Chhotabhai JaverBhai Patel College, RTM Nagpur University, Tirora-441911, Nagpur, India
- *Corresponding Author:
- Sangita D Katre
Department of Chemistry
Chhotabhai JaverBhai Patel College
RTM Nagpur University
Tirora-441911, Nagpur, India
Abstract
Although there are various oxidants known for their versatility & efficiency, still there is a continuous search for new oxidants in the field of organic synthetic chemistry. Over the past few years, a lot of work has been done on the utility of chromium(VI) based oxidants, leading to the development of a variety of reagents. The present study compiles different types of chromium complexes and their synthetic utility in organic chemistry as new oxidizing systems.
Keywords
Chromium(VI) reagents, Oxidation, Organic synthesis
Introduction
In recent years, oxidation processes have received much attention, especially in the search for selective and environmentally friendly oxidants [1,2]. Chromium(VI) based reagents have been extensively used as oxidizing agents in synthetic organic chemistry [3-8]. Many among these have become quite popular and proven to be potent oxidizing agents. These versatile reagents are capable of oxidizing almost every oxidizable organic functional group. The important ones among these Cr(VI) reagents include-ditertiary butyl chromate [9], tetrakis(pyridine) silver dichromate [10], tripropyl ammonium fluorochromate [11], quinolinium fluorochromate [12], quinolinium chlorochromate [13], 3,5-dimethyl pyrazolium fluorochromate [14], Collins reagent [15], chromium trioxide-3,5-dimethyl pyrazole complex [16], Pyridinium Chlorochromate (PCC) [17], Pyridinium Dichromate (PDC) [18], 2,2’-Bipyridinium Chlorochromate (BIPCC) [19], 2,6-dicarboxy pyridinium chlorochromate [20,21], N-Methyl piperidinium chlorochromate [22], Tetramethyl Ammonium Fluorochromate VI (TMAFC) [23], N-Methyl Benzyl Ammonium Fluorochromate (MBAFC) [24], Cetylrimethyl Ammonium Bromochromate (CTMABC) [25], pyridine chromium peroxide [26], Caffinilium chlorochromate [27], Isoquinolinium chlorochromate [28], Chromium trioxide 3,5-Dimethylpyrazole complex (CrO33.5-DMP) [29], quinolium chloromate [30], prolinium chlorochromate [31], tributyl ammonium chlorochromate [32], triphenyl methyl phosphonium chlorochromate [33].
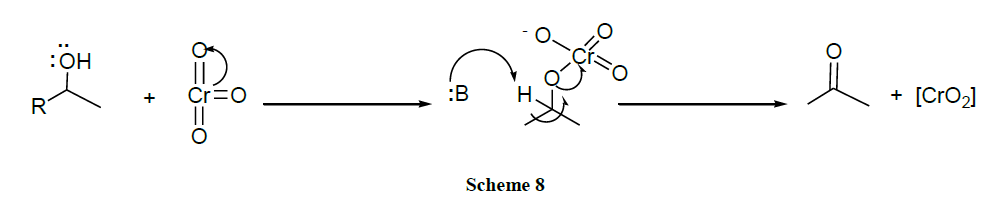
The first attempt to make a mild reagent of substituted chromates was reported by Sarett and coworkers, who used pyridine to form a salt with CrO3, in order to oxidize steroidal alcohols. A variety of Cr(VI) oxides derived from CrO3 were long among the most popular reagents for oxidation of alcohols to aldehydes and ketones. Pyridinium chlorochromate, pyridinium dichromate and chromic oxide-pyridine (Collins reagent) are the most common used ones. The oxidation of alcohols can usually be stopped at the aldehyde stage although oxidation to carboxylic acids can also be achieved.
Despite its selectivity, Collin’s reagent suffers from difficulties associated with its preparation, stability and efficiency. The less reactive adducts PCC and PDC are more easily handled and more selective than Collin’s reagent in oxidation of alcohols. These reagents as well as other more exotic adducts of nitrogen heterocycles with Cr(VI), facilitate a number of oxidative transformations of organic compounds, including cyclization to form tetrahydrofuran derivatives and allylic transposition to afford enones from allylic alcohols.
Many authors [34-37] have reviewed and reported the oxidation of organic compounds by Cr(VI) in acidic media. Cr(VI) oxidations of inorganic substrates was studied and reviewed by Beattie & Haight [38]. The chromates and dichromates of Cr(VI) are reported [39] to be highly soluble and very toxic as well. Therefore a continuous search for a selective and efficient oxidizing agent for various organic substrates under milder conditions was of interest to synthetic organic chemists. This led to the development of many such reagents with some success [40].
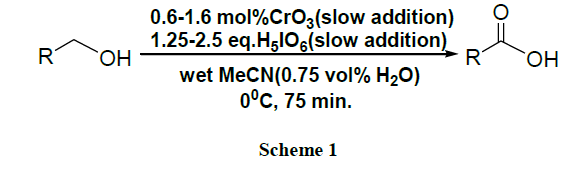
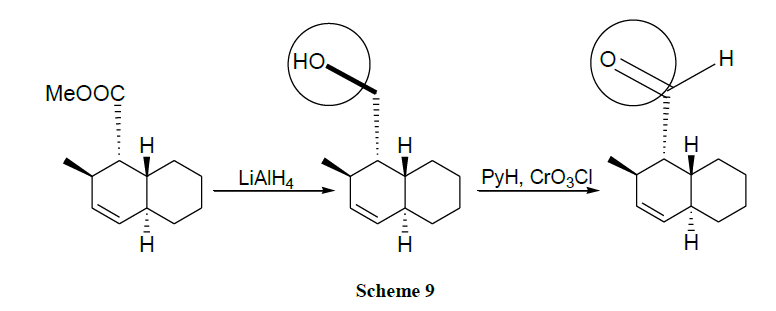
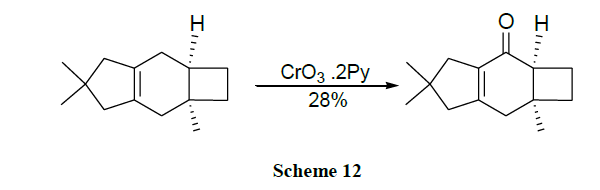
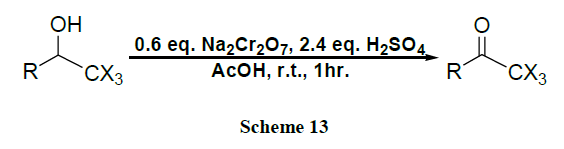
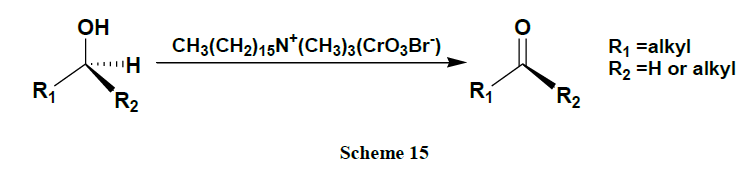
Oxidation of alcohols into the corresponding aldehydes and ketones efficiently using trimethyl-silyl chlorochromate was reported [41,42]. An excellent yield of carboxylic acids is obtained by CrO3 catalysed novel oxidation of primary alcohols by using only 1-2 mol % of CrO3 and 2.5 equivalents of H5IO6 in wet MeCN. Here, no significant racemization is observed for alcohols with adjacent chiral centres. However, secondary alcohols are cleanly oxidized to ketones (Schemes 1-17) [43].
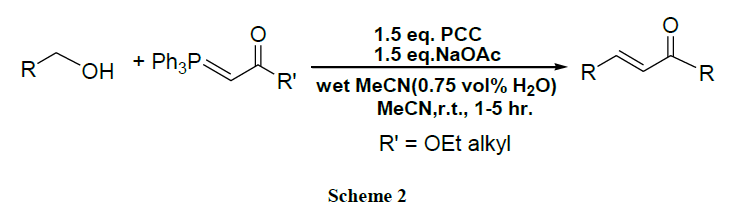
A domino oxidation of primary alcohols gives α, β-unsaturated compounds by using PCC-NaOAc and stabilized by Wittig reagents [44].
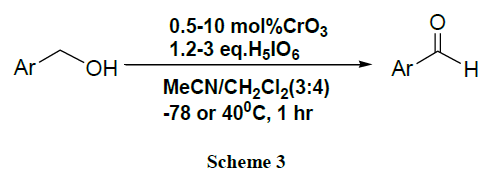
A CrO3 catalysed efficient oxidation of benzyl alcohols and benzyl TBDMS ethers to give high yield of corresponding carbonyl compounds proceeds with periodic acid at low temperature (-78°C). The oxidation procedure was found to be highly functional group tolerant & very selective for the TBDMS group over the TBDPS group [45].
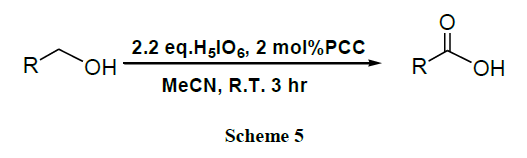
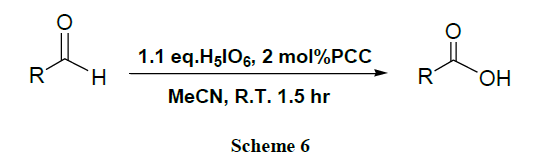
A facile and quantitative preparation of carboxylic acids by PCC catalyzed (2 mol %) oxidation of primary alcohols and aldehydes using 2.2 equivalents and 1.1 equivalents of H5IO6, respectively, in acetonitrile is described [46].
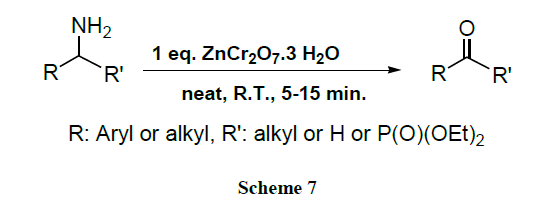
A potent and expeditious oxidative deamination of various α-aminophosphonates allows the synthesis of α-keto-phosphonates using ZnCr2O7.3H2O under solvent free conditions at room temperature [47]. This method can also be applied to the rapid and highly selective oxidation of various amines to aldehydes & ketones in very good yields.
The oxidation of alcohols by modified oxochromium(VI) amine reagents was reported [48,49].
Domino reaction of solanopyrone in total synthesis was described [50].
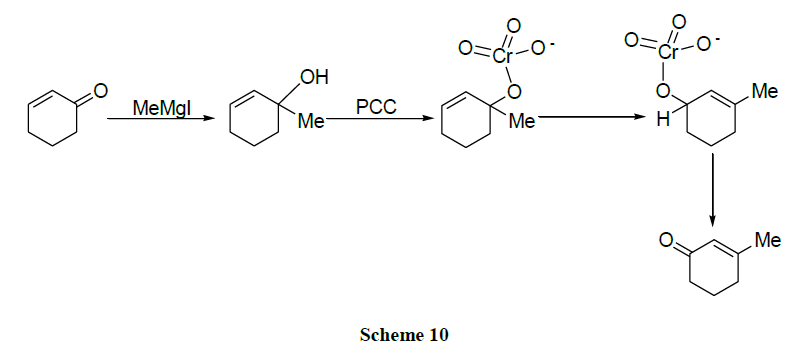
Majetich and coworkers [51] reported the oxidation of tertiary allylic alcohols to rearranged enones.
Chromium oxide reagents are used as a better option for the difficult transformation of the functionalization of allylic positions which are usually done by distinct methods like allylic halogenation, singlet oxygen and selenium dioxide). Numerous other reagents like NBS, SeO2, CrO2Cl2 failed altogether. Cr(VI) complexes oxidize alkenes to enones in favourable cases. The reaction is carried out efficiently when one of the double bond termini is fully substituted i.e., cannot be oxidized to a ketone [52,53].
Sarrett [54] identified the adduct of pyridine and Cr(VI) oxide i.e., Collin’s reagent as a selective compound for the oxidation of primary and secondary alcohols to carbonyl compounds. Trihaloacetic acids can be converted to trichloromethyl and tribromomethyl ketones in good yield by a catalyzed reaction with aldehydes followed by oxidation [55].
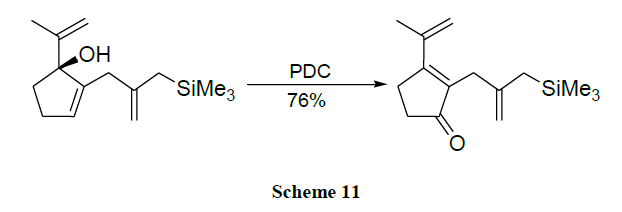
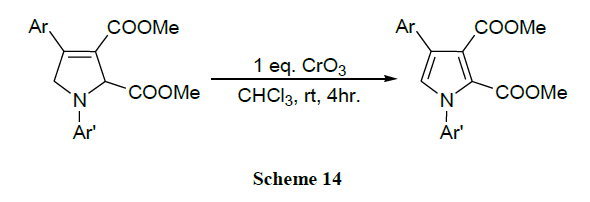
A reaction between dialkyl acetylene dicarboxylates and β-amino ketones promoted by triphenyl phosphine allows an efficient one pot synthesis of polysubstituted 2,5-dihydropyrrole derivatives. The prepared 2,5-dihydropyrroles can be easily oxidized to the corresponding pyrrole derivatives with chromium trioxide [56].
Two new mild oxidizing agents [57]. Tetramethylguanidium Halochromates (TMGFC & TMGCC) were prepared in high yields by reacting tetra methyl guanidine with CrO3 and related acid. These reagents are suitable to oxidize various primary & secondary alcohols and oximes to corresponding carbonyl compounds under solvent free conditions & microwave irradiation.
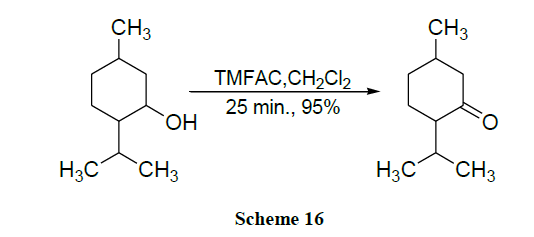
A simple and selective method for the oxidation of alcohols to carbonyl compounds using wet alumina supported ammonium chlorochromate in solventless system is described [58]. An efficient & mild method for the oxidation of alcohols and polyarenes is described using TMAFC under microwave irradiation [59].
Oxidation of lysine by chromium (VI) in acid perchlorate medium has been studied. Microwave assisted oxidation of organic compounds such as carbohydrates and polycyclic arenes by cetyltrimethyl ammonium chlorochromate was reported [60].
Conclusion
Cr(VI) complexes are considered as a very important class of organic compounds because of their oxidizing properties. Cr(VI) reagents have a wide spectrum of application in synthetic organic chemistry. Here we have made an effort to compile most of these oxidation processes using these reagents which are reported in recent years. However, there is still a need to explore several areas of these transition metal complexes and to synthesize new ones with more properties.
References
- R.S. Varma, R.K. Saini, Tetrahedron Lett., 1998, 39(12), 1481-1482.
- S. Patel, B.K. Mishra, Tetrahedron., 2007, 63(21), 4367-4406.
- W.J. Mijs, de Jonge, CRHI Eds. Plenum Press, New York, 1986, 68-81.
- Neeraj, A.K. Pandey, G.D. Mishra, J. Chemtracks., 2009, 11(2).
- S.V. Ley, A. Madin, Organic Syntheses, Pergamon Press, Oxford, 1991.
- G. Cainnelli, G. Cardillo, Chromium oxidations in organic chemistry, Springer Verlag, Berlin, 1984.
- M. Fieser, Reagents for Organic synthesis, Wiley-Interscience, New York, 1984, 11(450).
- R.V. Oppenauer, H. Oberrauch, (Univ. Innsbruck, Austria), Anaus Assoc. Guin-Argentina, 1949, 37, 246-262.
- H. Firouzabadi, A. Sardarian, H. Gharibi, Synth. Commun., 1984, 14, 89.
- S. Ghammamy, A. Hashemzadeh, Bull. Korean Chem. Soc., 2004, 25, 1277-1279.
- V. Murugesan, A. Pandurangan, Indian J. Chem. Sec. B., 1992, 31B, 377.
- R. Srinivasan, C.V. Ramesh, W. Madhulatha, K. Balasubramanian, Indian J. Chem. Sec. B., 1996, 35B, 480.
- U. Bora, M.K. Chaudhari, Tetrahedron., 2001, 57, 2445.
- J.C. Collins, W.W. Hess, F.J. Frank, Tetrahedron Lett., 1968, 3363.
- E.J. Corey, G.W. J. Fleet, Tetrahedron Lett., 1973, 45, 4499.
- E.J. Corey, J.W. Suggs, Tetrahedron Lett., 1975, 2647.
- E.J. Corey, G. Schmidt, Tetrahedron Lett., 1979, 399.
- F.S. Guziec, F.A. Luzzio, Synthesis., 1980, 691.
- M. Tajbakhsh, R. Hosseinzahed, M. Yazdani-Niaki, J. Chem. Res., 2002, 2.
- R. Hosseinzahed, M. Tajbakhsh, M. Yazdani-Niaki, Tetrahedron Lett., 2002, 43, 9413- 9416.
- M. Takbakhsh, M.M. Heravi, F. Mohanazadeeh, S. Sarabi, M. Ghassemzadeh, Montash. Chem., 2001, 132, 1229-1231.
- M.K. Kassaee, A.R. Mahjoub, S. Ghammami, Tetrahedron Lett., 2003, 44, 4555-4557.
- M.K. Kassaee, H. Sajjadi-Ghotbabadi, S.Z. Sayyed-Alangi, Pittcon., 2003, 1200-1207.
- H. Eimanieh, S. Ghammami, M.K. Mohammadi, Synth. Commun., 2007, 37, 601-607.
- H. Firouzabadi, N. Iranpoor, F. Kiaeezadeh, J. Toofan, Tetrahedron., 1986, 42(2), 719-725.
- F. Shirin, I. Mohammadpoor, Baltork, Z. Hejazi, P. Heravi, Bull. Korean Chem. Soc., 2003, 24, 517-518.
- R. Srinivasan, P. Stanley, K. Balasubramanian, Synth. Commun., 1997, 27, 2057-2064.
- E.J. Corey, G.W.J. Fleet, Tetrahedron Lett., 1973, 14, 4499-4501.
- G. Javanthi, G. Vijayakumar, K.P. Elango, J. Serb. Chem. Soc., 2002, 67, 803-808.
- M. Mamaghami, F. Shirini, F. Parsa, Russ. J. Org. Chem., 2002, 38, 1113-1115.
- S. Ghammami, S.A. Seyed Sadjadi, J. Serb. Chem. Soc., 2005, 70, 1243-1248.
- A.R. Hazipour, L. Khadooz, A.E. Ruoho, J. Iranian Chem. Soc., 2005, 2, 315-318.
- F.H. Westheimer, Chem. Rev., 1949, 45, 419.
- K.B. Wiberg, Oxidation in Organic Chemistry, Part A, Academic: New York, 1965.
- G. Cainelli, G. Cardillo, Chromium Oxidations in Organic Chemistry, Springer-Verlag: Berlin, 1984.
- A.K. Das, Oxid. Commun., 2001, 24, 321.
- J.K. Beattie, G.P. Haight Jr., Prog. Inorg. Chem., 1972, 17, 93.
- M.E. Losi, C. Amrhein, W.T. Frankenberger, Rev. Environ. Contam. Toxical., 1994, 136, 91-211.
- L.F. Fieser, M. Fieser, Reagents for Organic Synthesis, Wiley, New York, 1967-1984, 1-11.
- J.M. Aizpurua, C. Palomo, Tetrahedron Lett., 1983, 24, 4367.
- J.M. Aizpurua, B. Lecea, M. Juaristi, C. Palomo, Tetrahedron., 1985, 41, 2903.
- M. Zhao, J. Li, Z. Song, R. Desmond, D.M. Tschaen, E.J.J. Grabowski, P.J. Reider, Tetrahedron Lett., 1998, 39, 5323-5326.
- J. Shet, V. Desai, S. Tilve, Synthesis., 2004, 1859-1863.
- S. Zhang, L. Xu, M.L. Trudell, Synthesis., 2005, 1757-1760.
- M. Hunsen, Synthesis., 2005, 2487-2490.
- S. Sobhani, M.F. Maleki, Synlett., 2010, 382-386.
- F.A. Luzzio, Org. React.,1998, 53, 1-221.
- F.A. Luzzio, F.S. Guziec, Organic Preparations & Procedures International., 1998, 20(6), 533-584.
- H. Hagiwara, J. Org. Chem., 2002, 67, 5969.
- G. Majetich, J.S. Song, A.J. Leigh, S.M. Condon, J. Org. Chem., 1993, 58, 1030.
- M. Harmata, G. Bohnert, J. Org. Lett., 2003, 5, 59.
- J.M. Humphrey, Y. Liao, A. Ali, T. Rein, Y.L. Wong, H.J. Chen, A.K. Courtney, S.F. Martin, J. Am. Chem. Soc., 2002, 124, 8584-8592.
- G. Poos, G.E. Arth, R.E. Beyler, L.H. Sarrett, J. Am. Chem. Soc., 1953, 75, 422.
- E.J. Corey, J.O. Link, Y. Shao, Tetrahedron Lett., 1992, 33, 3435-3438.
- M. Anary-Abbasinejad, E. Poorhassan, A. Hassanabadi, Synlett., 2009, 1929-1932.
- K. Sendil, H.B. Ozgun, E. Ustun, J. Chem., 2016, 351802.
- M.M. Heravi, M.M. Aghayan, Zfur Naturforschung., 2014, 54(6), 815-817.
- M.K. Mohammadi, S. Ghammami, H. Imaneih, Bull. Chem. Soc. Ethiop., 2008, 22(3), 449-452.
- S. Mathur, M.B. Yadav, V. Devra, Int. J. Chem. Sci., 2015, 13(2), 641-649.
- M.K. Mohammadi, Open J. Synthesis Theory & Application., 2013, 2, 87-90.