Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Application of RP-HPLC and UV-Spectrophotometric Method in Dissolution Testing of Teneligliptin
Atul T Hemke*, Yogesh G Ghuge and Krishna R Gupta
Department of Pharmaceutical Chemistry, Smt. Kishoritai Bhoyar College of Pharmacy, New Kamptee, Nagpur, Maharashtra, 441002, India
- Corresponding Author:
- Atul T Hemke
Department of Pharmaceutical Chemistry
Smt. Kishoritai Bhoyar College of Pharmacy, New Kamptee
Nagpur, Maharashtra, 441002, India
Abstract
Characterization of drug product performance in the pharmaceutical field has become very essential; Dissolution testing has emerged as an important tool. Teneligliptin is mainly used for the treatment of type-II diabetes mellitus, not official in any of the monographs. The objective of the study was to develop and validate UV spectrophotometric and RP-HPLC methods for dissolution testing for the quality control of Teneligliptin tablets. In vitro dissolution tests of tablets were performed using different trial conditions. The satisfactory sink conditions (Test conditions) includes phosphate buffer of pH 7.5 (900 ml at 37 ± 0.5°C) as dissolution medium, USP Apparatus II (paddle method) with agitation speed of 50 rpm. The method was found to be linear with correlation coefficient (r2=0.999) in the concentration range of 10-60 μg/ml. The chromatographic separation was achieved on Shodex C18 column with isocratic mode using a mixture of methanol: phosphate buffer (pH 7.2) in the ratio of 70:30 v/v, flow rate of 1 ml/min and detection wavelength was 245.6 nm. The recovery of the drug at three levels was found to be nearly equal to 100%. The proposed dissolution test method is adequate and can be applied for the quality control of 20 mg Teneligliptin tablets.
Keywords
Teneligliptin (TENE), Validation, Dissolution testing, RP-HPLC
Introduction
Teneligliptin is a highly potent, competitive and long-lasting DPP-4 inhibitor mainly used for the treatment of Type-II Diabetes Mellitus (T2DM) acts by improving postprandial hyperglycemia and dyslipidemia (Figure 1).
Recent studies have suggested that Teneligliptin (TENE) exhibits multiple pharmacological effects that include vasoprotective and neuroprotective effects also. Literature reveals some analytical methods have been reported for the estimation of TENE in formulation and plasma [1-4]. The proposed work describes development of simple, precise and economic UV spectrophotometric and High Performance Liquid Chromatography (HPLC) method for estimation of TENE. In recent years, pharmaceutical industry and regulatory authorities are emphasizing on dissolution testing of formulations. To assure product quality and performance after the manufacturing process of a drug product, changes in the formulation can be assessed easily through dissolution tests from lot-to-lot during the development of new formulations [5]. TENE is not official drug in any of the Pharmacopoeia and also no dissolution test method was found in literature. Hence, objective of current study was to develop an accurate and precise dissolution test method for product performance.
Material and Methods
Chemicals and reagents
Teneligliptin in salt form (TEHH) was procured from Glenmark Pharmaceutical, Ltd, (Sinnar, India). The commercially formulation of Teneligliptin were purchased form Indian market. Chemicals include methanol and acetonitrile of HPLC grade, potassium dihydrogen phosphate; Ortho phosphoric acid, hydrogen chloride and sodium hydroxide of GR grade were used. 0.1 N HCl, acetate buffer pH 4.0, Phosphate Buffer pH 6.8 and phosphate buffer pH 7.5 were prepared as per the pharmacopoeia.
Instruments
Dissolution Apparatus: Electrolab, Tablet Dissolution tester (TDT) 06P, Lab India Ds1400, HPLC: Shimadzu HPLC series 1100, UVSpectrophotometer: Jasco V-630, Shimadzu-1700 double beam, Sonicator: PCI Mumbai 3.5L 100H, Spectra lab. UCB-300, pH-meter: GLOBAL Model No. DPH-500, EI, Model No. 1102012 and Weighing Balance: Shimadzu AUX220, RADWAG PS1500
Development of UV-spectrophotometric method
Standard stock solution
An accurately weighed about 10.0 mg of TENE was transferred in a 10.0 ml volumetric flask, dissolved in methanol to prepare a stock standard solution of 1000 μg/ml of TENE.
Working standard solution
1.0 ml of the standard stock solution was diluted to 10.0 ml (100 μg/ml of TENE); from this solution further 3.0 ml was diluted to 10.0 ml to prepare working standard solution having concentration about 30 μg/ml of TENE.
Selection of wavelength
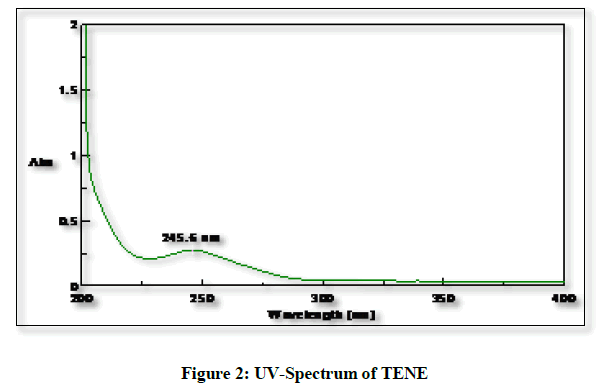
The working standard solution prepared above of TENE (30 μg/ml) was scanned in the UV range of 200-400 nm in 1.0 cm cell against solvent blank (methanol) and spectrum recorded (Figure 2). The recorded spectrum of TENE showed peak maxima at 245.6 nm and 280 nm. The wavelength of 245.6 nm was considered as detection wavelength for further experimentation.
Development of RP- HPLC method
Standard stock solution
Transfer an accurately weighed quantity of TEHH is equivalent to TENE (~10.0 mg) was in a 10.0 ml volumetric flask, dissolved and volume make up with diluents to prepare standard stock solution of 1000 μg/ml of TENE.
Working standard solution
The working stock solution was further appropriately diluted with mobile phase to get the final concentration of 30 μg/ml of TENE.
Phosphate buffer (pH 7.2)
Transfer 50.0 ml of 0.2 M potassium dihydrogen phosphate in 200.0 ml volumetric flask, to its 34.7 ml of 0.2 N NaOH was added and volume make up with water, sonicated and filtered through 0.4 μm membrane filter paper.
Development of dissolution test method
Determination of solubility and sink conditions
Solubility profile was used as the basis for the selection of a dissolution medium for TENE. Drug solubility was determined at 25°C in different media and expressed as mg/ml. Sink conditions were determined in different media.
Mechanical calibration of dissolution apparatus
Conventionally, for oral solid dosage forms, dissolution Apparatus I or II is suggested by FDA guideline but to satisfy with cGMP requirements mechanical calibration for Apparatus I and II should be carried out.
Optimization of dissolution test
The dissolution studies were performed using a six-station dissolution apparatus by subjecting six commercial formulation in each dissolution medium containing 900 ml of dissolution media using both a paddle and basket dissolution apparatus and stirring speeds of 50, 75 and 100 rpm at temperature 37 ± 0.5°C were tried [6-8]. Aliquots of 10 ml were withdrawn manually at intervals of 5, 10, 15, 20 and 25 min. The same volume of fresh medium at 37 ± 0.5°C was replaced to maintain the constant volume. The sample was filtered through Whatman filter paper and analyzed by UV and RP-HPLC method.
Validation of dissolution method
The proposed method was validated for its specificity, linearity, accuracy, precision and robustness to demonstrate reproducibility and reliability [9-10].
Linearity
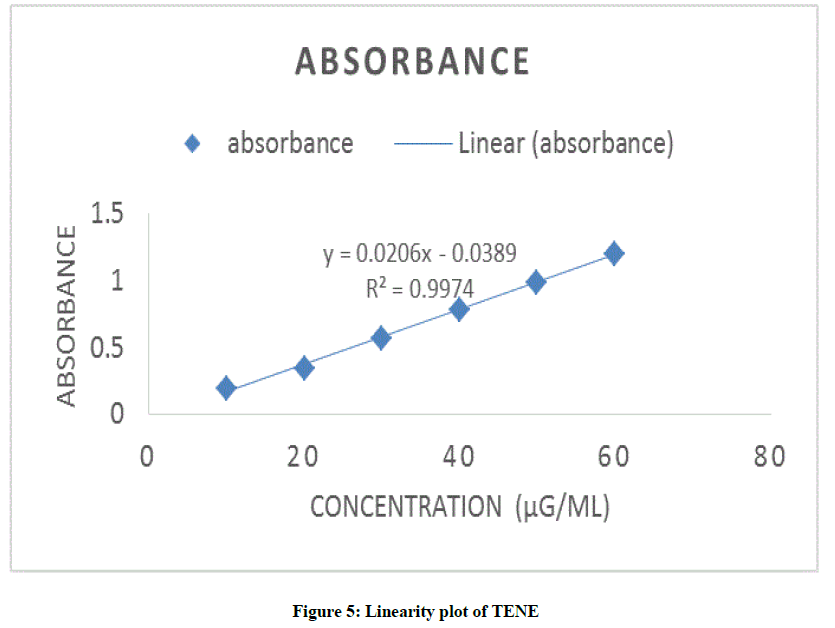
Aliquots of TENE stock solution (100 μg/ml) were diluted with phosphate buffer pH 7.5 to give concentrations of 10-60 μg/ml. Each solution was read in triplicate. Calibration curve was plotted as absorbance/AUC Vs concentration.
Precision
Using the optimized dissolution parameters, test solutions were obtained and the test solution was withdrawn at 5 min time interval upto 25 min. The absorbance and area under curve was noted to estimate the amount of drug release at each time interval using proposed methods. Thus, repeatability was evaluated at the 100% level and the Relative Standard Deviation (RSD) of the data was calculated. The evaluation of intermediate precision was performed by analysing the sample on different days by different analyst, and the %RSD values were calculated.
Accuracy
The accuracy of proposed method was carried out by performing recovery study for TENE; standard drug substance was added to the dissolution vessels in known amounts at the 80%, 100% and 120% levels. Accordingly, 11.2, 14 and 16.8 mg of standard drug was added along with 20.0 mg tablet. Dissolution test was carried out for 25 min using 900.0 ml of phosphate buffer pH 7.5 as dissolution medium in paddle type-II apparatus at 50 rpm (Temp. 37 ± 0.5°C). Aliquots of 10.0 ml were withdrawn at appropriate interval and filtered through Whatman filter paper and analysed by UV and RP-HPLC.
Robustness of the test method
The robustness of analytical method is the ability to remain unaffected by small but deliberate variations in method parameters and provide an indication of its repeatability during normal uses. The robustness study was performed for change in flow rate and wavelength.
Results and Discussion
Teneligliptin was found to highly soluble and stable in methanol, distilled water and mixture of methanol: phosphate buffer (pH 7.2). Using these solvents, working standard solutions were prepared of desired concentration for UV and HPLC estimation of TENE.
Optimised chromatographic conditions
In order to achieve the optimized chromatographic condition, one or two parameter modified at each trial and chromatograms were recorded with all specified chromatographic conditions. The various mobile phases were tried to select the most suitable one by changing flow rate, buffer and its pH (Table 1).
| HPLC System | Shimadzu HPLC series 1100 |
| Column | Shodex C-18-4E (5 µm), 250 × 4.6 mm |
| Mobile phase | Methanol: Phosphate buffer pH 7.2 (70:30% v/v) |
| Detection wavelength | 245.6 nm |
| Flow rate | 1.0 ml/min |
| pH | 7.2 |
| Injection volume | 20 µl |
Table 1: Optimized chromatographic conditions
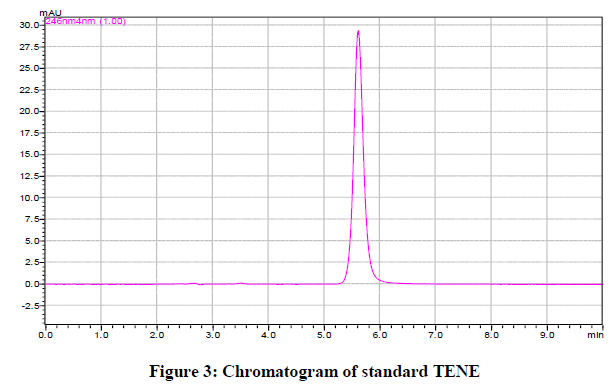
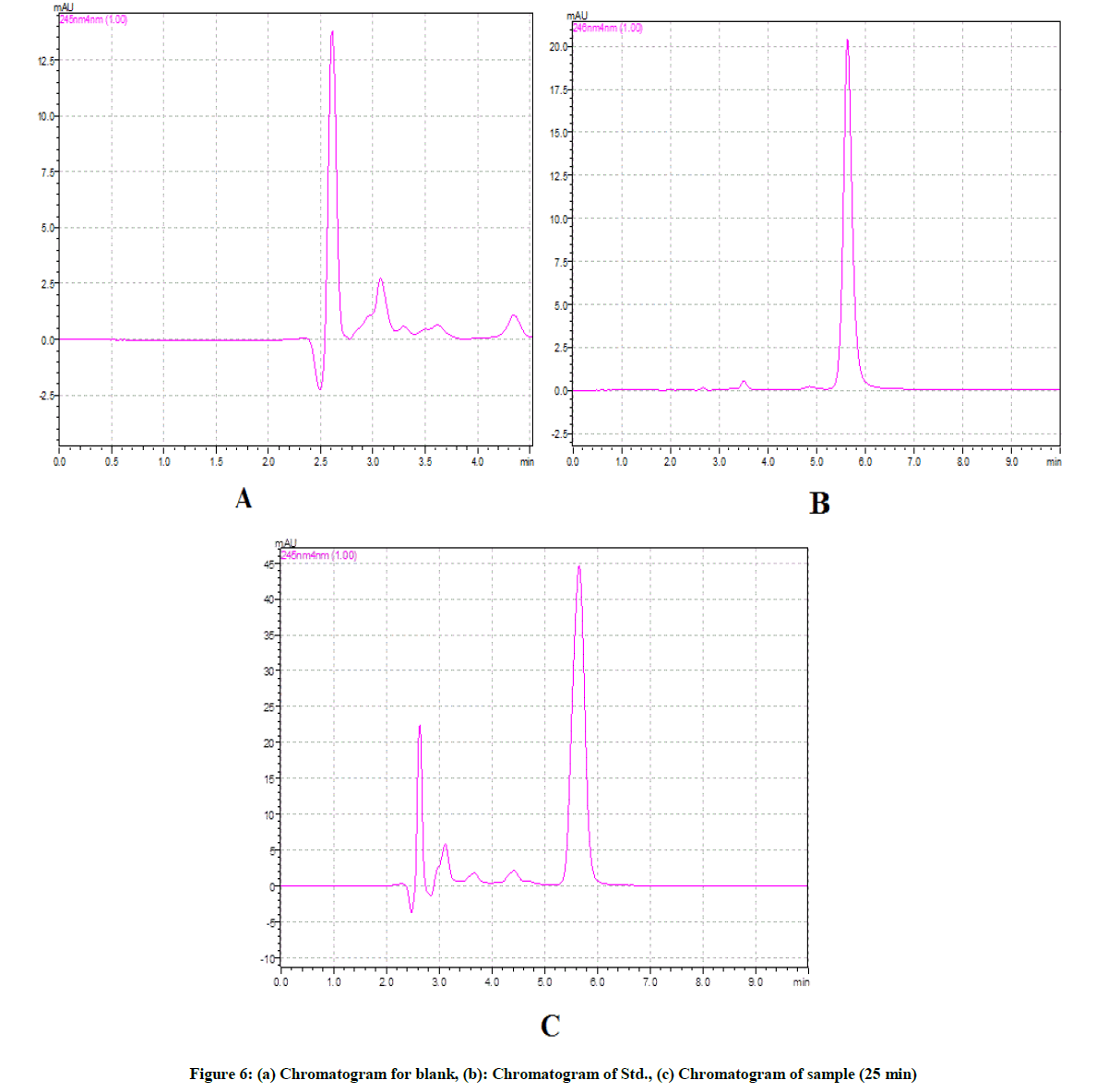
The chromatographic conditions were set as per final chromatographic conditions; mobile phase was allowed to equilibrate with stationary phase as indicated by steady baseline. The mobile phase containing Methanol: Phosphate buffer, pH 7.2 (70:30% v/v) gave well resolved peak and reasonable retention time as shown in Figure 3.
System suitability parameters
The system suitability study was performed by injecting six replicate injection of standard TENE (30 μg/ml) were injected and chromatographed. The results obtained indicate that proposed method was suitable for further experimentation (Table 2).
| Sr. No. | Wt. of Std. drug taken (mg) | Area (µV) |
|---|---|---|
| 1 | 10.03 mg | 760561 |
| 2 | 769738 | |
| 3 | 779503 | |
| 4 | 768035 | |
| 5 | 771992 | |
| 6 | 775843 | |
| Mean | 770945.33 | |
| ± SD | 6573.86 | |
| % RSD | 0.85 | |
| Retention time | 5.615 | |
| Tailing factor (Asymmetry) | 0.956 | |
| Theoretical plate | 16720.50 | |
Table 2: Results of system suitability parameters
Drug substance solubility study
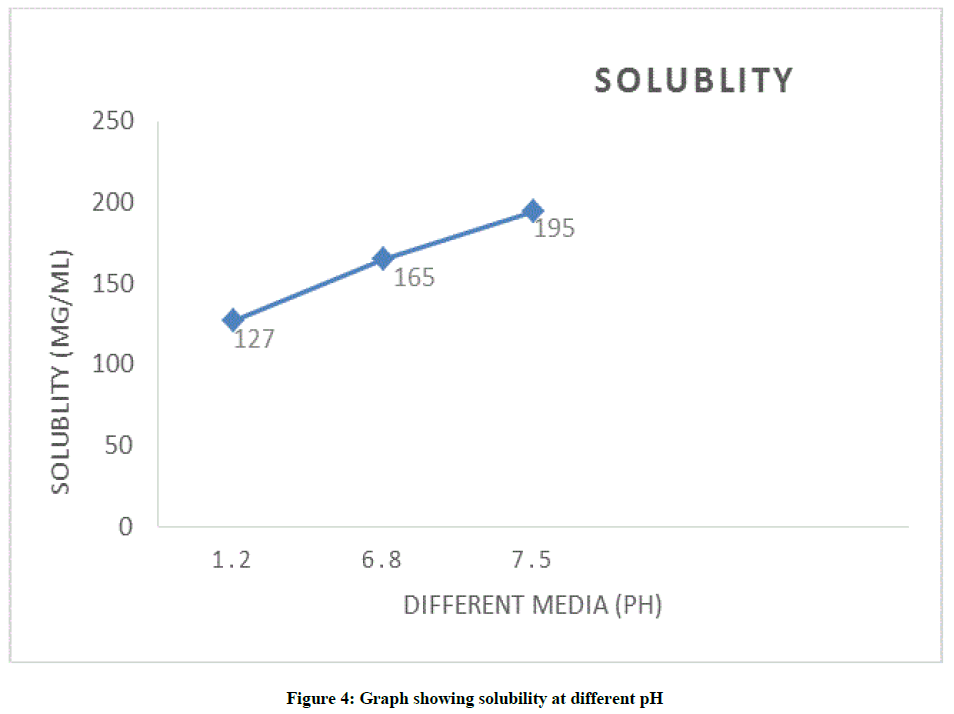
Solubility study of TENE was carried out by using different dissolution media. The solution was sonicated, filtered and analysed by UV spectroscopy to determine the solubility of the drug in respective dissolution media. The graph was plotted between pH of dissolution media and observed solubility (Figure 4).
Optimization of dissolution parameters
Various dissolutions were performed to optimized dissolution parameter. For maximum percent release of drug, trials were taken by using USP Apparatus I and II, i.e., Basket and paddle type at different rpm 50, 75 and 100. Based on the solubility of TENE, phosphate buffer pH 7.5 was selected as suitable buffer media as compared to Acetate buffer pH 4.0 and Phosphate buffer pH 6.8 (Tables 3 and 4).
| Phosphate Buffer pH 7.5 as dissolution media | Time points (min) % release | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | ||
| Type of apparatus | USP type-I | 19.28 | 28.97 | 42.57 | 66.77 | 73.20 |
| Speed of rotation | 100 RPM | |||||
Table 3: Results showing % release of drug using USP type-I basket apparatus
| Phosphate Buffer pH 7.5 as dissolution media | Time points (min) % release | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | ||
| Type of apparatus | USP type-II | 6.59 | 21.59 | 33.43 | 56.13 | 72.80 |
| Speed of rotation | 50 RPM | |||||
Table 4: Results showing % release of drug using USP type-II paddle apparatus
was observed that the release of drug in USP type-I (Basket) apparatus, shows maximum release at first time point. Using type-II apparatus, proper profiling for drug release was observed (Table 5).
| Phosphate buffer pH 7.5 as dissolution media | Time points (min) % release | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | ||
| Type of Apparatus | USP type-II | 14.27 | 22.81 | 35.39 | 69.98 | 72.84 |
| Speed of rotation | 75 RPM | |||||
Table 5: Results showing effect of change in speed of rotation on TENE
The release of drug at first time point was more when the speed of rotation was changed from 50 rpm to 75 rpm i.e. very fast release was observed at 75 rpm as compared to 50 rpm using type-II apparatus. The following dissolution parameters have been finalized for the estimation of TENE as depicted in Table 6.
| Drug | Dissolution media | Media volume | USP apparatus | RPM |
|---|---|---|---|---|
| TENE | Phosphate buffer pH 7.5 | 900.0 ml | Type-II paddle | 50 |
Table 6: Final optimized dissolution conditions
Linearity
Pipette out 1.0-6.0 ml from the working stock solution (100 μg/ml) and diluted with the buffer upto 10.0 ml in separate volumetric flasks. Absorbance of each solution and peak area was noted. Calibration curve was plotted as absorbance/AUC Vs concentration as shown in Figure 5. The obtained result shows linearity between concentration and absorbance/AUC.
Percent release of Teneligliptin
The test solution was obtained by performing the dissolution of drug under finalised dissolution parameters. The six replicates of test solution of drug so obtained were chromatographed (Figure 6) and %RSD of drug was calculated and recorded in Table 7. The proposed RP-HPLC method was found to be precise with %RSD 1.30.
| Test sample | Peak area (µV) | % Dissolution of TENE at 25 min |
|---|---|---|
| 1 | 1043682 | 70.33 |
| 2 | 1047465 | 70.58 |
| 3 | 1068373 | 71.99 |
| 4 | 1072983 | 72.23 |
| 5 | 1076389 | 72.53 |
| 6 | 1059784 | 72.09 |
| Mean | 10611446 | 71.625 |
| ± SD | 13551.15 | 0.9277 |
| %RSD | 1.28 | 1.30 |
Table 7: Observations and results of precision study
Intermediate precision
Estimation of TENE in marked formulation analyzed by proposed methods had yield quit concurrent results, standard deviation and %RSD of series of measurement were found to be within limit (Not more than 2%) (Tables 8 and 9).
| Average % release ± SD (n=3) | ||||
|---|---|---|---|---|
| S. No. | Time (min) | 10:00 am | 01.00 pm | 04.00 pm |
| 1. | 5 | 7.29 ± 0.004 | 6.83 ± 0.0062 | 8.68 ± 0.00264 |
| 2. | 10 | 24.02 ± 0.0198 | 23.17 ± 0.01358 | 26.76 ± 0.0125 |
| 3. | 15 | 59.09 ± 0.0160 | 57.26 ± 0.01058 | 61.06 ± 0.00513 |
| 4. | 20 | 66.95 ± 0.00321 | 66.34 ± 0.00643 | 67.63 ± 0.00814 |
| 5. | 25 | 71.17 ± 0.00513 | 71.49 ± 0.004 | 73.49 ± 0.00458 |
| Average at 25 min | 72.05 ± 0.00457 | |||
| %RSD | 1.66 | |||
Table 8: Dissolution test precision (Intraday) results
| Average % release ± SD (n=3) | ||||
|---|---|---|---|---|
| Sr. No. | Time (min) | 1st day | 2nd day | 3rd day |
| 1. | 5 | 4.88 ± 0.00551 | 4.51 ± 0.00321 | 8.57 ± 0.00321 |
| 2. | 10 | 28.52 ± 0.007 | 28.51 ± 0.00361 | 34.33 ± 0.00651 |
| 3. | 15 | 47.60 ± 0.00551 | 48.45 ± 0.00115 | 54.57 ± 0.0052 |
| 4. | 20 | 60.92 ± 0.00351 | 60.78 ± 0.003 | 68.97 ± 0.00379 |
| 5. | 25 | 71.05 ± 0.00351 | 71.54 ± 0.00251 | 72.67 ± 0.0153 |
| Average at 25 min | 71.75 ± 0.007106 | |||
| %RSD | 1.56 | |||
Table 9: Dissolution test precision (Interday) results
The %RSD for dissolution of the test sample of TENE was found to be 1.66 and 1.56 which is within the acceptance limit.
Accuracy of test method
The accuracy study for Teneligliptin was demonstrated by adding standard drug substance to the dissolution vessel in known amounts at the 80%, 100% and 120% levels. Accordingly about 11.2 mg, 14.0 mg and 16.8 mg of reference drug was added along with 20.0 mg tablet. Dissolution test was performed for 25 min using 900.0 ml of phosphate buffer pH 7.5 as a dissolution medium in a paddle type at 50 rpm. Aliquots of 10.0 ml were withdrawn and filtered through Whatman filter paper and analyzed by proposed methods (Table 10).
| S. No. | Accuracy level | % Recovery* | |
|---|---|---|---|
| UV | RP-HPLC | ||
| 1. | 80% | 97.08 | 99.45 |
| 2. | 100% | 98.84 | 100.70 |
| 3. | 120% | 100.26 | 101.42 |
| Mean | 98.72 | 100.52 | |
| ± SD | 1.59 | 0.99 | |
| %RSD | 1.61 | 1.01 | |
Table 10: Results of recovery study
The data indicated that %RSD values and the mean % recovery found against added amount was under acceptance criteria.
Robustness of test method
The robustness of the proposed method was evaluated by change in analyst and instrument using optimized dissolution parameters (Table 11).
| Avg. % release ± SD (n=3) | |||||
|---|---|---|---|---|---|
| S. No. | Time (min) | Analyst | Instrument | ||
| I | II | I | II | ||
| 1. | 5 | 8.92 ± 0.00656 | 9.54 ± 0.00666 | 6.08 ± 0.002193 | 7.52 ± 0.0231 |
| 2. | 10 | 20.05 ± 0.005 | 18.33 ± 0.01217 | 27.67 ± 0.00231 | 25.09 ± 0.00755 |
| 3. | 15 | 41.27 ± 0.01413 | 41.18 ± 0.00379 | 55.67 ± 0.00651 | 58.20 ± 0.00701 |
| 4. | 20 | 49.25 ± 0.00866 | 49.93 ± 0.01044 | 69.19 ± 0.01015 | 70.27 ± 0.0164 |
| 5. | 25 | 74.07 ± 0.00503 | 74.29 ± 0.00208 | 74.92 ± 0.00751 | 75.22 ± 0.00346 |
| Avg. % release at 25 min | 74.18 ± 0.003555 | 75.065 ± 0.005485 | |||
| % RSD | 1.15 | 1.77 | |||
Table 11: Results of robustness study
The %RSD of dissolution study for TENE was found to 1.15 and 1.77, which is within the limit of acceptance limit. Also robustness study by RP-HPLC method was carried out via change in flow rate (± 0.2 ml/min.) and detection wavelength (± 5 nm). The overall %RSD for the deliberate variations was found within the range.
Conclusion
The proposed dissolution test method for Teneligliptin by using UV spectrophotometer and RP-HPLC was developed and validated as per the ICH guidelines. The result obtained by proposed method was found to be reliable, accurate and precise. Hence, the developed methods can be employed for routine dissolution analysis of Teneligliptin tablets.
Acknowledgement
The authors would like to thankful to Principal of Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, Nagpur (MS), India for providing us research facility.
References
- M. Kishimoto, Diabetes Metab. Syndr. Obes., 2013, 6(20), 187-195.
- S. Reddy, K. Saraswathi, Int. J. Adv. Pharm. Res., 2014, 310-318.
- T. Yoshida, F. Akahoshi, H. Sakashita, Bio. Med. Chem., 2012, 20(19), 5705-5719.
- S. Fukuda, J. Anabuki, Euro J. Pharmacol., 2012, 9-24.
- L.C. Vaucher, Quim. Nova., 2009, 32(5), 1-5.
- P.A. Patel, V.B. Patravale, Int. J. Pharm. Sci. Res., 2010, 1(8), 282-292.
- A.R. Breier, C.S. Paim, M. Steppe, J. Pharm. Pharm. Sci., 2005, 8(2), 289-298.
- I.E. Shohin, J.I. Kulinich, G.V. Ramensakya, G.F. Vasilenko, Disso. Technol., 2011, 18(1), 26-29.
- Validation of Analytical Procedures: Text and Methodology, (Q2R1); ICH Harmonized Tripartite Guideline: Geneva, Switzerland, 2005.
- USP 32–NF 27; The United States Pharmacopoeial Convention, Inc.: Rockville, MD, 2009.