Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 5
Antimicrobial Studies of Essential Oils of some Medicinal Plants against Multi Resistant Acinetobacter Strains
Soheir S Abdel Salam1*, Nahed M Ayaat1, Mahmoud M Amer1, Ahmed A Shaheen2 and Hend E Amin1
1Microbiology Department, Faculty of Science, Benha University, Egypt
2Microbiology Department, Faculty of Medicine, Zagazig University, Egypt
- *Corresponding Author:
- Soheir S Abdel Salam
Microbiology Department
Faculty of Science
Benha University, Egypt
Abstract
A big problem in intensive care units (ICU) is caused by antibiotic resistant bacterial nosocomial infections. Acinetobacter are among the most challenging bacterial pathogens and to help in formulating antibiotic policy for better management of patients with bacterial diseases. The data showed that 93.3% of bacterial isolates were resistant to ampicillin while 80% and 66.7% of bacterial isolates were resistant to ceftazidime and sulphamethoxazole/trimethoprim, respectively. Identification of multi-resistant isolates according to morphological and biochemical characteristics, Acinetobacter baumannii found to be the most frequent pathogen representing 66.7% followed by Acinetobacter calcoaceticus and Acinetobacter lwoffii with 20% and 13.3% percentages, respectively. The main objective of this study was studying the susceptibility of multi-drug resistant isolates to different ten essential oils derived from different parts of ten medicinal plant species traditionally used in Egyptian folk medicine. The essential oils of Clove, Thyme and Rosemary were the most active oils respectively. Followed by Marjoram, Black seed, Lemon grass, Fennel, Pepper mint, Chamomile and Anise respectively. The identification of the most frequent and multi-drug resistant Acinetobacter baumannii isolate (5) was confirmed by using 16S Ribosomal Ribonucleic Acid (rRNA) gene.
Keywords
Intensive care units, Antibiotic resistance, Essential oils, Antimicrobial activity, Acinetobacter sp.
Introduction
Nosocomial infections (NIs) are frequent complications of hospitalizations. The quality of health care and a principal source of adverse outcomes are critical problems, which have been affected by nosocomial [1]. Currently, the clinicians are facing the most challenging bacterial pathogens which are Acinetobacter species. Acinetobacter spp. demonstrate high rates of resistance to multiple antimicrobial agents [2]. Acinetobacter baumannii, Acinetobacter calcoaceticus, Acinetobacter haemolyticus and Acinetobacter lwoffii are the most important species in clinical practices [3]. Acinetobacter spp. cause a wide range of health care-associated infections such as: ventilator-associated pneumonia, bloodstream infections, urinary tract infections, surgical site infections, meningitis, cholangitis, peritonitis, skin and wound infections, ventriculitis and infective endocarditis [4]. Widespread use of antimicrobials within hospitals resulted to the emergence and increase of antibiotics resistance among Acinetobacter strains, in particular, the wide use of extended-spectrum cephalosporins and quinolones. Acinetobacter species are more resistance to antimicrobial agents than other representatives from the family Enterobacteriaceae [5-8].
Unlike antibiotics, which are chemotherapeutic drugs used, mostly, internally to control infections with specific structures [9]. Treatment of infections continues to be a problem in modern time because of severe side effects of some drugs and growing resistance to antimicrobial agents. Hence, search for newer, safer and more potent antimicrobials is a pressing need [10]. The plant essential oils are rich source of scents and used in food preservation and aromatherapy. These possess multiple antimicrobial (antibacterial, antifungal & antiviral), anticancer and antioxidant properties [11-14]. Essential oils and extracts from aromatic plants have long been used for a wide variety of medicinal and domestic purposes. The combined effect of both their active and inactive compounds were the result of their action, several active components might have synergistic effect [11,15]. Some plant extracts were more active than commercial antibiotics [16]. The activity of chamomile (Matricaria chamomilla) toward all of the Aerobacter strains tested was significantly enhanced by using methanol as a solvent [17]. Oil extracted from chamomile was active in low concentrations against Streptococcus pyogenes, Streptococcus mutans, Streptococcus salivarius and Streptococcus faecalis [18]. Black seed used as a traditional medicine for treatment of various diseases such as skin infection [19]. The essential oil of the seeds has antibacterial effects on Gram-positive and Gram-negative bacteria [20]. Cymbopogon citratus (Lemon grass) has quite high degree of antimicrobial activity against oral pathogen [21]. Essential oil of clove showed strong antibacterial activity against Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa and Klebsiella pneumoniae [22]. Ethanolic extracts of clove showed inhibitory activity against all the six food associated bacteria [23]. Rosmarinus officinalis essential oil has immense medicinal worth for its powerful antimutagenic, antiphlogistic, antioxidant, chemo preventive and antibacterial properties [24]. Thyme has been extensively used as herbal tea, tonic, carminative, antitussive, expectorant, and antiseptic, as well as for treating colds [25]. Thyme oil considered as potent food preservative due to its antimicrobial properties, the mode of action of essential oils and extracts on bacterial cells. They can degrade the cell wall, disrupt the cytoplasmic membrane, cause leakage of cellular compounds, change fatty acids and phospholipids constituents and influence the synthesis of Deoxyribonucleic Acid (DNA), Ribonucleic Acid (RNA) and cell protein [26,27]. The aim of the work, studying the antibacterial effect of some famous medicinal plants essential oils used in Egypt to introduce new alternatives against multi-drug resistant Acinetobacter infections.
Materials and Methods
Clinical specimens
Samples were collected during a period from May to December 2013 from Zagazig University hospitals. Under aseptic conditions, the samples were collected from patients with different infections [28]. Clinical samples were investigated to find the distribution of nosocomial pathogens in causing different opportunistic infections and their antibiotic resistance profile.
Antibiotic susceptibility tests
Ten different antibiotics were selected for carrying out the antimicrobial susceptibility test. The antibiotic disks (Oxoid Ltd., England) were placed onto the surface of the inoculated agar medium and then plates were incubated at 30°C for 48 h and the standard evaluation of inhibition zones according to [29]. The antibiotics which were tested were Amikacin 30 μg (AK), Ampicillin 10 μg (AM), Ampicillin/sulbactame 10/10 μg (SAM), Ceftazidime 30 μg (CAZ), Ciprofloxacin 5 μg (CIP), Gentamicin 10 μg (GM), Imipenem 10 μg (IPM), Polymyxin B 300 μg (PB), Tetracycline 30 μg (Te) and Sulphamethoxazole/ trimethoprim 23.75/1.25 μg (SXT).
Isolation, purification and Identification of bacterial isolates
The inoculated and streaked Mac Conkey agar plates were incubated at 37°C/24 h. Selected bacterial isolates were streaked for several consecutive times on nutrient agar medium until pure single colonies were obtained. The purified selected isolates were identified morphological characters and biochemical tests as Gram staining, capsule staining, motility, oxidase and catalase tests according to [30,31].
Antibacterial activity of essential oils against the selected multi-drug resistant Acinetobacter strains
Different concentrations of volatile oils (diluted by twin 80) from medicinal plants shown in Table 1 were tested against the selected multi resistant Acinetobacter isolates using disc diffusion method according to [13,32]. By using sterilized Muller-Hinton agar medium and inoculated with the multi resistant. The density of the bacterial suspension equivalent to that of standard barium sulphate (0.5 McFarland) is determined. Sterile filter paper discs were saturated by diluted volatile oils, the disks were allowed to dry for one hour and then placed on the surface of agar plates and incubated for 24 h at 37ºC then, the diameter of the inhibition zone was measured including the diameter of the disk (6 mm).
| Family | Scientific name | Arabic name | Parts used |
|---|---|---|---|
| Asteraceae (Compositae) | Matricaria chamomilla | بابونج | Flowers |
| Lamiaceae (Labiatae) | Origanum vulgare | بردقوش | Aerial parts |
| Lamiacea (Labiatae) | Mentha piperita | نعناع فلفلي | Aerial parts |
| Lamiaceae (Labiatae) | Rosmarinus officinalis | إكليل الجبل | Aerial parts |
| Lamiaceae (Labiatae) | Thymus vulgaris | زعتر | Aerial parts |
| Apiaceae (Umbelliferae) | Pimpinella anisum | ينسون | Seeds |
| Apiaceae (Umbelliferae) | Foeniculum vulgare | شمر | Seeds |
| Myrtaceae | Syzygium aromaticum | قرنفل | Floral buds |
| Poaceae (Gramineae) | Cymbopogon citratus | حشيشة الليمون | Whole plant |
| Ranunculaceae | Nigella sativa | حبة البركة | Seeds |
Table 1: Family, scientific, English, Arabic names and parts used from each plant in preparing essential oils
Molecular identification of the most resistant and frequent strain
By investigation of 16S Ribosomal ribonucleic acid (rRNA) gene sequences, identification of the most resistant and frequent strain, A. baumannii (5), was confirmed. The genes coding for (16S rRNA) are referred to as 16S Recombinant Deoxyribonucleic acid (rDNA) and are used in phylogenetic studies [33]. The 16S rDNA gene was amplified by polymerase chain reaction (PCR) using universal primers, 8F (5'- GAGTTTGAT CCTGGCTCAG-3') and 1492R (5'-CGGTTACCTTGTTACGACTT-3').
Statistical analysis
Statistical comparisons between the groups were performed using the one-way analysis of variance (ANOVA). Data were expressed as means, Student’s f-test and using the one-way Analysis of Variance (ANOVA) in SPSS® Statistics software. All parameters were considered significantly different if P ˂ 0.05.
Results and Discussions
Distribution of collected isolates
A total number 955 samples were collected from different medical specimens of different patients (Male & female), total collected samples (580) produced positive growth while 375 samples produced negative growth. Acinetobacter spp. bacteria isolated from respiratory tract infections represented by 46.7% of total Acinetobacter spp. isolates, while those from urinary tract infections, wound infections, blood stream infections represented by 26.7%, 13.3% and 13.3%, respectively Table 2. These results are in line with that of [34] who demonstrated that 584 Acinetobacter strains isolated from 420 patients at 12 different hospitals over a 12 mon period, 426 (72.9%) strains were identified as A. baumannii, with 208 A. baumannii isolates being recovered from respiratory tract specimens, 113 being recovered from blood cultures and central venous lines, 70 being recovered from wound swabs.
| Source of isolation | No. | % |
|---|---|---|
| Respiratory tract infections | 7 | 46.7 |
| Urinary tract infections | 4 | 26.7 |
| Wound infections | 2 | 13.3 |
| Blood stream infections | 2 | 13.3 |
| Total Acinetobacter spp. isolates | 15 | 100 |
Table 2: Distribution of Acinetobacter spp. isolates according to source of isolation
Antibiotic susceptibility tests
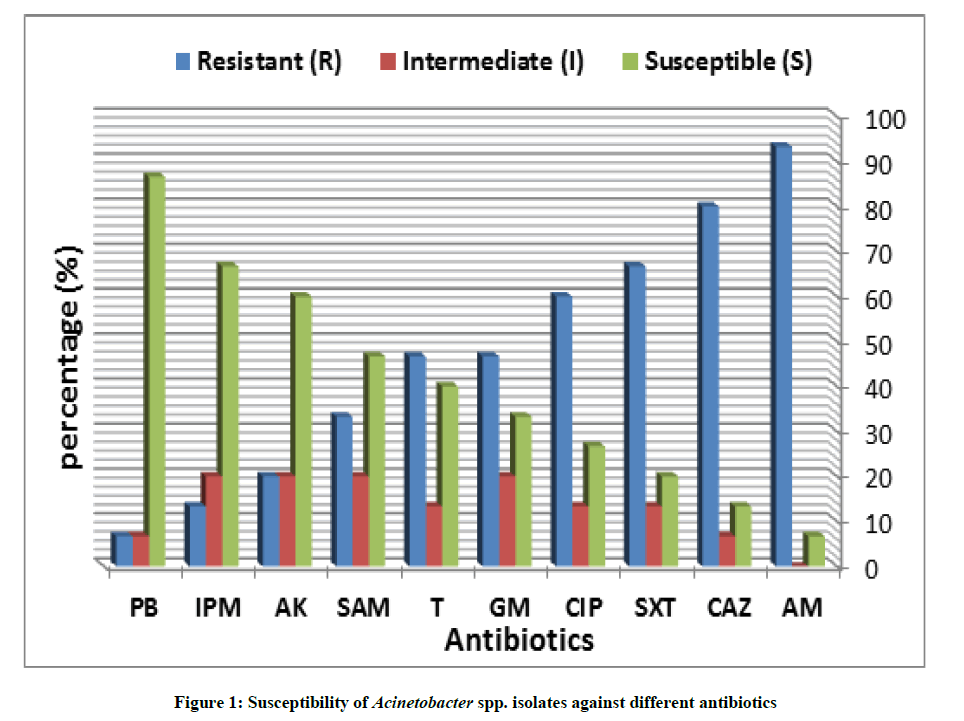
This experiment was carried out to study the susceptibility of the different bacterial isolates towards different ten antibiotics by using a standardized disc diffusion method. The results in Figure 1 revealed that the tested isolates were highly susceptible to polymyxin B with susceptibility percentage (86.6%) so that it represented the most effective antibiotic followed by imipenem, amikacin, ampicillin/sulbactame and tetracycline with 66.7%, 60%, 46.7% and 40% susceptibility, respectively. On the other hand the data showed that 93.3% of bacterial isolates were resistant to ampicillin while 80% and 66.7% of bacterial isolates were resistant to Ceftazidime and Ulphamethoxazole/Trimethoprim, respectively.
Identification of Acinetobacter spp. by morphological, physiological and biochemical tests
Different morphological, physiological and biochemical tests were conducted to identify multidrug resistance of Acinetobacter spp. isolates. The obtained results are tabulated in Table 3. According to the keys of identification protocols the tested isolates were divided into three groups as following:
| Test | Group I | Group II | Group III |
|---|---|---|---|
| Morphological characters | |||
| Gram's stain | - ve | - ve | - ve |
| Shape | Coccobacilli | Coccobacilli | Coccobacilli |
| Colonies characters | circular, convex, smooth, and slightly opaque with entire margins; colonies are 0.5-1.5 mm in diameter after 24 h and 2.5-3.5 mm in diameter after 48 h | circular, convex, smooth, and slightly opaque with entire margins and sometimes have a butyrous aspect. Colonies are 1.5-2.0 mm in diameter after 24 h and 3.0-4.0 mm in diameter after 48 h | circular, convex, smooth, and slightly opaque with entire margins; colonies are 1.0-1.5 mm in diameter after 24 h and 3.0-4.0 mm in diameter after 48 h |
| Motility | - ve | - ve | - ve |
| Growth at 44°C | + ve | - ve | - ve |
| Physiological characters | |||
| Oxidase | - ve | - ve | - ve |
| Catalase | + ve | + ve | + ve |
| Coagulase | - ve | - ve | - ve |
| Blood hemolysis | - ve | - ve | - ve |
| Indole | - ve | - ve | - ve |
| Methyl Red | - ve | - ve | - ve |
| Voges-Proskauer | - ve | - ve | - ve |
| Citrate | + ve | + ve | - ve |
| H2S production | - ve | - ve | - ve |
| Nitrate reduction | - ve | - ve | - ve |
| Gelatin Liquification | - ve | - ve | - ve |
| Acid from | |||
| Glucose | + ve | + ve | - ve |
| Galactose | + ve | + ve | - ve |
| Lactose | + ve | + ve | - ve |
| Xylose | + ve | + ve | - ve |
| Sucrose | - ve | - ve | - ve |
| D-Malate | + ve | - ve | - ve |
| Glycerate | - ve | + ve | - ve |
| Identification | Acinetobacter baumannii | Acinetobacter calcoaceticus | Acinetobacter lwoffii |
Table 3: Morphological characteristics, biochemical tests and confirmatory tests for identification of the suspected Acinetobacter spp. Isolates
Group I: Acinetobacter baumannii
Group II: Acinetobacter calcoaceticus
Group III: Acinetobacter lwoffii
Antibacterial activity of selected essential oils against the Multi-Drug Resistant (MDR) Acinetobacter strains
Essential oils have been traditionally used for treatment of infections and diseases all over the world for centuries. In recent years there has been extensive research to explore and determine the antimicrobial activity of essential oils. Previous studies have showed that plant essential oils possess the capacity to inhibit microorganisms [35,36]. Also essential oils are aromatic and volatile oily liquids obtained from plant materials as secondary metabolites. They are normally found in leaves, stems, barks and fruits [37]. Thymol, carvacrol, linalool and eugenol are main constituents of some plant essential oils that have been shown to have a wide spectrum of activity against microbes [38].
The present study determined the antibacterial activity of ten essential oils in Tables 4-6. The results revealed that all Acinetobacter isolates are sensitive to clove even at low concentration. This match with [39] who found that clove oil was active against Gram-negative bacteria. This due to eugenol, the major constituent of clove oil; eugenol exhibits pharmacological effects on almost all systems in the body. Eugenol possesses significant antioxidant and anti-inflammatory properties, in addition to having analgesic and local anesthetic activity [40].
| Essential oils | Chamomile | Anise | Pepper mint | Thyme | Marjoram | Clove | Lemon grass | Black seed |
|---|---|---|---|---|---|---|---|---|
| 20% | 0d | 0d | 0d | 0e | 0e | 0e | 0c | 0d |
| 40% | 0d | 0d | 0d | 9d | 8d | 10d | 0d | 0d |
| 60% | 8c | 7c | 8c | 16c | 13c | 18c | 10c | 12c |
| 80% | 12b | 10b | 10b | 20b | 16b | 22b | 15b | 15b |
| 100% | 15a | 13s | 13a | 24a | 21a | 25a | 19a | 20a |
| L.S.D | 0.45 | 0.3 | 0.45 | 1.87 | 0.98 | 1.91 | 0.91 | 0.86 |
Means with same letters within column non-significant difference; Means with different letters within column significant difference, P ≤ 0.05-0.01; L.S.D.: Least significant difference at P ≤ 0.05-0.01
Table 4: Antibacterial activity of selected essential oils against Acinetobacter baumannii (5)
| Essential oils | Chamomile | Anise | Pepper mint | Thyme | Marjoram | Clove | Lemon grass | Black seed |
|---|---|---|---|---|---|---|---|---|
| 20% | 0d | 0d | 0e | 0e | 0e | 9e | 0e | 0e |
| 40% | 0d | 0d | 8d | 12d | 10d | 12d | 7d | 10d |
| 60% | 11c | 10c | 10c | 19c | 15c | 21c | 12c | 13c |
| 80% | 15b | 14b | 13b | 22b | 18b | 26b | 18b | 17b |
| 100% | 17a | 17a | 17a | 28a | 24a | 30a | 22a | 23a |
| L.S.D | 0.55 | 0.48 | 0.51 | 1.88 | 95 | 2.21 | 0.97 | 1.72 |
Means with same letters within column non-significant difference; Means with different letters within column significant differenc, P ≤ 0.05-0.01; L.S.D.: Least significant difference at P ≤ 0.05-0.01
Table 5: Antibacterial activity of selected essential oils against Acinetobacter calcoaceticus (12)
| Essential oils | Chamomile | Anise | Pepper mint | Thyme | Marjoram | Clove | Lemon grass | Black seed |
|---|---|---|---|---|---|---|---|---|
| 20% | 0d | 0d | 0d | 0e | 0e | 0e | 0e | 0d |
| 40% | 0d | 0d | 0d | 11d | 8d | 12d | 7d | 0d |
| 60% | 8c | 9c | 9c | 18c | 13c | 19c | 11c | 12c |
| 80% | 12b | 12b | 13b | 22b | 16b | 22b | 16b | 15b |
| 100% | 15a | 15a | 15a | 26a | 22a | 29a | 19a | 21a |
| L.S.D | 1.03 | 0.84 | 0.47 | 1.86 | 0.93 | 1.89 | 1.03 | 0. 97 |
Means with same letters within column non-significant difference; Means with different letters within column significant difference, P ≤ 0.05-0.01; L.S.D.: Least significant difference at P ≤ 0.05-0.01
Table 6: Antibacterial activity of selected essential oils against Acinetobacter lwoffii (2)
Rosemary was active against A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). Our results match with [41] who reported that rosemary plants are rich sources of phenolic compounds with high antimicrobial activity against both Gram-positive and Gram-negative.
Lemon grass had high effect on A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). Our result match with [42] who reported that lemon grass showed high inhibitory effect on Gram-negative and does not match with [43].
Marjoram oil was active against A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). The present results were in agreement with [44] showed that concentrations of marjoram oil for gram-negative bacteria were lower than gram-positive bacteria. Moreover it was reported that volatile aromatic components in plant kingdoms exhibit more antimicrobial potential than those of components non aromatic volatile essential oils [45]. Anise had intermediate effect against A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). These results go in line with [46] as gram-negative bacteria. Thyme had higher effect on all tested bacterial isolates. Our results are in agreement with [47] as gram-negative bacteria. Peppermint oil had intermediate effect on the growth of all bacterial isolates due to the active constituents in peppermint oil, which was prepared through distillation of the ground parts of the peppermint plant, include methanol, menthone, cineol, and several other volatile oils [48]. Nigella sativa (Black seed) was active against A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). The antibacterial activity to rosemary oil may due to one of the active ingredients was Thymoquinone (volatile oil of these seeds) and Melanin (fixed oil). This result match with [49] as gram-negative bacteria. Fennel had significant antibacterial activity against A. baumannii (5), A. calcoaceticus (12) and A. lwoffii (2). This result go in the line with [50,51] as gram-negative bacteria. But don’t match with [52] who reported that Gram-negative strains of bacteria have less sensitivity to fennel essential oil. Chamomile oil had intermediate effect on the growth of all bacterial isolates and this result match with [53] who illustrated antimicrobial activity may be due to numerous free hydroxyl ions that have the capability to combine with the carbohydrates and proteins in the bacterial and fungal cell wall they may get attached to enzyme sites rendering them inactive.
Molecular identification of the most frequent and multi-drug resistant Acinetobacter baumannii isolate
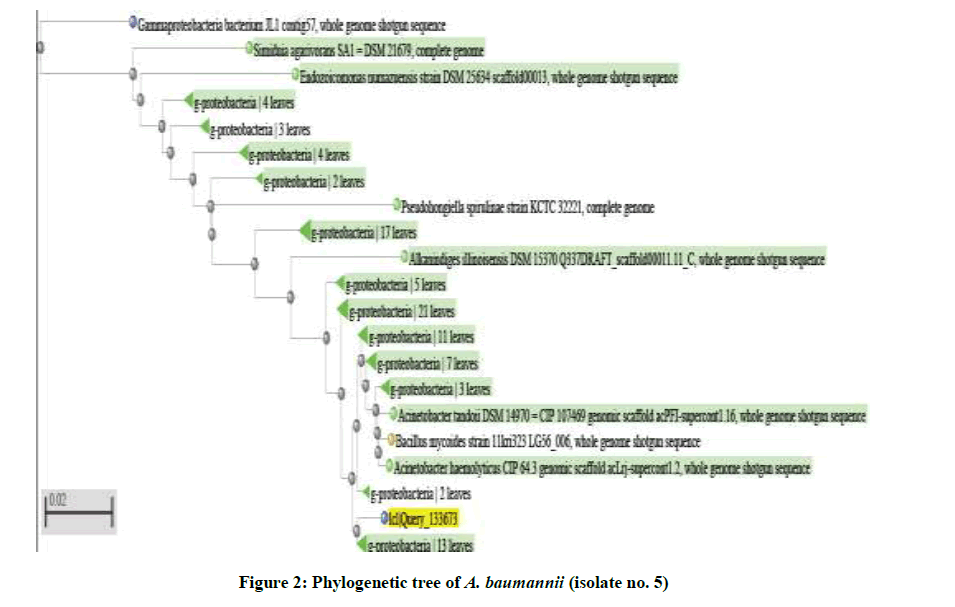
The identification of the most frequent and multidrug-resistant A. baumannii isolate (5) were confirmed by using 16S rDNA sequencing. Sequences datawere submitted to GenBank at NCBI web site (www.ncbi.nlm.nih.gov) with determined accession numbers. This was carried out using Sequencing Technology: Sanger dideoxy sequencing using specific universal forward and reverse primers. The 16S rRNA gene was amplified by Polymerase Chain Reaction (PCR) using universal primers designed to amplify 1.5 K base pair fragment of the conserved 16s rDNA region [54]. Purification and cycle sequencing also occurred. Sequence analysis and comparison to published sequences (based on alignment of 16s rDNA available in gene bank) made using web site http://blast.ncbi.nlm.nih.gov/ Blast.cgi commonly shown as phylogenetic trees or dendrogram (to visualize the result of a hierarchical clustering calculation) and linear alignments [55]. Results confirmed that the multidrug resistant bacteria isolates were A. baumannii (5) as gram-negative bacteria (Table 7 and Figure 2).
| Description | Max score | Total score | Ident | Accession |
|---|---|---|---|---|
| Acinetobacter baumannii strain XH386, complete genome | 1234 | 3234 | 98% | NZ_CP010779.1 |
| Acinetobacter haemolyticus CIP 64.3 genomic scaffold acLrj-supercont1.2, whole genome shotgun sequence | 1184 | 1184 | 97% | NZ_KB849805.1 |
| Acinetobacter calcoaceticus PHEA-2 chromosome, complete genome | 1179 | 2325 | 97% | NC_016603.1 |
| Acinetobacter junii CIP 64.5 genomic scaffold acLZZ-supercont1.1, whole genome shotgun sequence | 1175 | 2353 | 97% | NZ_KB849653.1 |
| Acinetobacter oleivorans DR1, complete genome | 1173 | 2999 | 97% | NC_014259.1 |
| Acinetobacter schindleri CIP 107287 genomic scaffold acLsx-supercont1.18, whole genome shotgun sequence | 1157 | 1157 | 96% | NZ_KB849580.1 |
| Acinetobacter parvus DSM 16617 = CIP 108168 genomic scaffold acLZw-supercont1.4, whole genome shotgun sequence | 1154 | 1861 | 96% | NZ_KB849210.1 |
| Acinetobacter sp. Ver3 contig00147, whole genome shotgun sequence | 1151 | 1151 | 96% | NZ_JFYL01000147.1 |
Table 7: Sequences producing significant alignments to isolate (No. 5)
Conclusion
The obtained results clearly demonstrated that all bacterial isolates showed resistance to essential oils in low concentrations but are sensitive to high concentration. The essential oils of Clove, Thyme and Rosemary were the most active oils respectively. Followed by Marjoram, Black seed, Lemon grass, Fennel, Pepper mint, Chamomile and Anise respectively.
References
- K. Ozdemir, M. Dizbay, J. Micro. Inf. Dis., 2015, 5(1), 38-43.
- P. Visca, H. Seifert, K.J. Towner, IUBMB Life., 2011, 63(12), 1048-1054.
- S.B. Almasaudi, Saudi J. Biol. Sci., 2016.
- M.E. Falagas, E.A. Karveli, I. Kelesidis, T. Kelesidis, Eur. J. Clin. Microbiol. Infect. Dis., 2007, 26(12), 857-868.
- F. Imperi, L.C. Antunes, J. Blom, L. Villa, M. Iacono, et al., IUBMB Life., 2011, 63(12), 1068-1074.
- L. Poirel, R.A. Bonnin, P. Nordmann, IUBMB Life., 2011, 63(13), 1061-1067.
- D.M. Livermore, Int. J. Antimicrob. Agents., 2012, 39(4), 283-294.
- S. Janahiraman, M.N. Aziz, F.K. Hoo, S.H. P’ng, Y.L. Boo, Pak. J. Med. Sci., 2015, 31(6), 1383-1388.
- A. Bridier, R. Briandet, V. Thomas, F. Dubois-Brissonnet, J. Bioadhesion and Biofilm Res., 2014, 27(9), 1017-1032.
- B.S.F. Bazzaz, M. Khajehkaramadin, H.R. Shokooheizadeh, Ir. J. Parma. Res., 2005, 4(2), 87-91.
- M.J. Chavan, D.B. Shinde, S.A. Nirmal, Nat. Prod. Res., 2006, 20, 754-757.
- J.C. Matasyoh, Z.C. Maiyo, R.M. Ngure, R. Chepkorir, Food Chem., 2009,113, 526-529.
- K. Zomorodian, P. Ghadiri, M.J. Saharkhiz, M.R. Moein, P. Mehriar, et al., Jundishapur J. Microbiol., 2015, 8(2), e17766.
- H.G. Mohamed, A.M. Gaafar, A.Sh. Soliman, Res. J. Micro., 2016, 11, 28-34.
- P. S. Negi, Int. J. Food Microbiol., 2012, 156, 7-17.
- A. Samie, C.L. Obi, P.O. Bessong, L. Namrita, Afr. J. Biotech., 2005, 4 (12), 1443-1451.
- L. Cervenka, I. Peskova; E. Foltynova; M. Pejchalova, I. Brozkova, J. Vytrasova, Current Microbiology, 2006, 53, 435-439.
- P. Owlia, I. Rasooli, H. Saderi, Res. J. Biol. Sci., 2007, 2(2), 155-160.
- M.N. Dang, M. Takacsova, D.V. Nguyen, K. Kristianova, Nahrung/Food., 2001, (45), 64 -66.
- S.J. Hosseinimehr, F. Pourmorad, N. Shahabimajd, K. Shahrbandy, R. Hosseinzadeh, Pak. J. Biol. Sci., 2007, 10, 637-640.
- M.L. Go-Yoshimura, S. Theerathavaj, T. Sroisiri, C. Suwan, 2007.
- S. Saeed, P. Tariq, Pak. J. Bot., 2008, 40(5), 2157-2160.
- R. Kumar, P. Jain, Sh. Chetan, Ethnobotanical Leaflets., 2010, 14, 344-360.
- N.M.A. Bousbia, M.A. Vian, E. Ferhat, B.Y. Petitcolas, F. Chemat, Food Chem., 2009, (15), 355-362.
- Z. Maksimovic, D. Stojanovic, I. Sostaric, Z. Dajic, M. Ristic, J. Sci. Food Agr., 2008, 88, 2036-2041.
- B. Shan, Y.Z. Cai, J.D. Brooks, H. Corke, Int. J. Food Microb., 2007, 117, 112-119.
- B.K. Tiwari, V.P. Valdramidi, C.P. O'Donnell, K. Muthukumarappan, P. Bourke, P.J. Cullen, J. Agr. Food Chem., 2009, 57, 5987-6000.
- P.R. Murray, E.J. Baron, J.H. Jorgensen, M.L. Landry, M.A. Pfaller, Manual of Clinical Microbiology, 9th Edi., ASM Press, Washington, DC, 2007.
- CLSI - Clinical and Laboratory Standards Institute, Performance standards for Antimicrobial susceptibility testing, 18th informational supplement, M100-S18, Wayne, PA, 2008.
- G.M. Garrity, D.J. Brenner, N.R. Krieg, J.T. Staley, Bergey’s Manual of Systematic Bacteriology, 2nd Edi., The Proteobacteria. Part B: The Gammaproteobacteria, Springer, New York, 2005, 2.
- C.R. Mahon, D.C. Lehman, G. Manuselis, Textbook of diagnostic microbiology, 4th Edi., W.B Saunders Co., Philadelphia, PA, 2011.
- Gulluce, S.J. Hosseinimehr, F. Pourmorad, N. Shahabimajd, K. Shahrbandy, Pak. J. Biol. Sci., 2007, 10, 637-640.
- W.G. Weisburg, S.M. Barns, D.A. Pelletier, D.J. Lane, J. Bacteriol., 2001, 173, 697-703.
- H. Seifert, R. Baginsky, A. Schulze, G. Pulverer, Zentralbl. Bakteriol., 1993, 279, 544-552.
- A.G. Ponce, C. Valle, S.I. Roura, J. Food Sci., 2004, 69, 50-56.
- W.X. Du, C.W. Olsen, R.J. Avena-Bustillos, T.H. Mchugh, C.E. Levin, J. Food Sci., 2009, 74, 390-397.
- M. Oussalah, S. Caillet, L. Saucier, M. Lacroix, Food Control., 2006, 18, 414-420.
- J.L. Rios, M.C.J. Recio, Ethanopharmacol., 2005, 6, 80.
- P. Lopez, C. Sanchez, R. Battle, C. Nerin, J. Agric. Food., 2005.
- K. Pramod, S.H. Ansari, J. Ali, Nat. Prod. Commun., 2010.
- P.R. Moreno Murray, E.J. Baron, J.H. Jorgensen, M.L. Landry, M.A. Pfaller, Manual of Clinical Microbiology, 9th Edi., ASM Press, Washington DC, 2007.
- B.S. Kruthi, K. Kruthi, P.S. Priya, T.H. Jyothi, S. Gogte, Inter. J. Scientific and Research Publications., 2012, 4(2), 1-8.
- M.F. Ghaly, M.A. Shalaby, Sm. M.S. Shash, M.N. Shehata, A.A. Ayad, J. App. Sci. Res., 2009, 5(10), 1298-1306.
- R.D.I. Pasqa, V.D.E. Feo, F. Villani, G. Mauriello, Ann. Microbiol., 2005, 55(2), 139-143.
- S.Y. Wang, P.F. Chen, S.T. Chang, Bioresource Technology., 2005, 96, 813-818.
- A. Akhtar, A.A. Deshmukh, A.V. Bhonsle, P.M. Kshirsagar, M.A. Kolekar, Veterinary World., 2008, 1(9), 272-274.
- S. Nanasombat, P. Wimuttigosol, Food Science and Biotechnology., 2011, 20(1), 45-53.
- M. Blumenthal, Herbal medicine: Expanded commission E Monographs 1st Edi., Newton, Mass: Integrative Medicine communications, 2000.
- J. Roy, D. Shaklega, P. Callery, J. Thomas, Afr. J. Tradit. Complement. Altern. Med., 2006, 3(2), 1-7.
- M. Araque, L.B. Rojas, A. Vsubillage, Science., 2007, 15(3), 366-370.
- A. EL-Adly, E.A. Abada, F.A. Charib, Int. J. Agric. Bio., 2007, 9(1), 22-26.
- L.P. Cantore, S.N. Lacobllis, G.F. Marco, F. Senatore, J. Agric. Food Chem., 2004, 52, 7862-7866.
- T. Santhamari, P. Meenakshi, S. Velayutham, 2011.
- C.T. Sacchi, A.M. Whitney, L.W. Mayer, R. Morey, A. Steigerwalt, Emerg. Infect. Dis., 2002, 8, 1117-1123.
- C.R. Woese, E. Stackebrandt, T.J. Macke, G.E. Fox, Syst. Appl. Microbiol., 1985, 6, 143-151.