Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 10
Antimicrobial Activities of Some Mesalazine Sulfonamides
Selvakumar Dineshkumar1 and Ganesamoorthy Thirunarayanan2*
1Department of Chemistry, Mahendra Arts and Science College, Kalippatti, Namakkal-637501, Tamilnaadu, India
2Department of Chemistry, Annamalai University, Annamaliangar-608002, Tamilnadu, India
- *Corresponding Author:
- Ganesamoorthy Thirunarayanan
Department of Chemistry
Annamalai University
Annamaliangar-608002, Tamilnadu, India
Abstract
About nine number of mesalazine sulfonamides were synthesized by zeolite Y clay modified copper nitrate catalyzed solvent-free condensation of various substituted phenyl sulfonyl chloride and 5-amino salicylic acid under microwave irradiation. Their purities were examined by their physical constants, analytical and spectroscopic data. The antimicrobial activities of these sulfonamides have been studied using Bauer-Kirby disc-diffusion method.
Keywords
Sulfonamides, Antimicrobial activities, Disc-diffusion.
Introduction
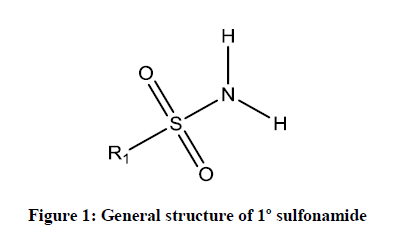
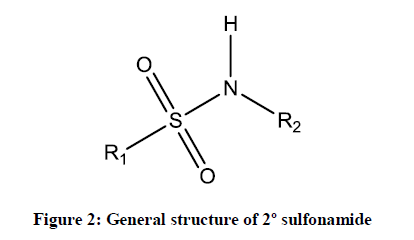
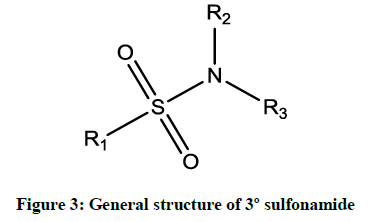
Sulfonamides are the first agents formulated drug for the treatment of bacterial infections in the 1930’s. Actually, this sulfonamide was discovered in 1936 by Gerhard Domagk (1895-1965), who found that an industrial dye named as Prontosil was able to cure infections in mice. He subsequently treated his daughter who was very ill with a streptococcal infection that failed to respond to standard treatments and won the Nobel Prize in 1938 for the discovery. Because Adolf Hitler prohibited German scientists from accepting this Nobel prize, he declined it but was given the award (without the prize money) in 1947, for his investigations of the activity of the Prontosil against streptococci [1]. Sulfonamides are widely used as antibiotics in the world. These are employed in clinical use since 1968. More than 30 sulfonamide based clinical drugs have been used commercially [2]. It is also been called as sulfa drugs. Sulfonamides or sulphonamide are effective compounds combining (–S(=O)2– NH–) group and they are generally formed by the condensation of corresponding amine and sulfonyl chlorides. The most common method for synthesizing sulfonamides is the condensation of aromatic sulfonyl chloride with aliphatic or aromatic primary/secondary amines in the presence of base catalysts. Usually sulfonamides are derived from amines with sulfonic acids or sulfonyl halides by eradicating of halide group or hydroxyl group and form important class of sulfur holding sulfonamides. The formed sulfonamides are generally primary, secondary and tertiary sulfonamides as shown in Figures 1-3.
The R1 and R2 are usually represented by alkyl, aryl or hydrogen and R3 is commonly hydrogen substitution in sulfonamide derivatives. Mesalazine (5-aminosalicylic acid) is also used to treat anti-inflammatory diseases and ulcerative colitis [1]. Many sulfonamide derivatives show a huge number of medicinal activities, such as anti-inflammatory, anti-cancer, antiviral agents [2], antimicrobial [3], antitumor [4], antithyroid [5] and Carbonic anhydrase inhibitors [6]. Recent literature review shows that, there are no reports available for the antimicrobial activities of these sulphonamides. Therefore, the authors interested to study the synthesis and antimicrobial activities of these sulphonamides. In this investigation the biological activities such as antibacterial and antifungal activities off these sulfonamide derivatives have been studied using the standard Bauer-Kirby [7] disc diffusion method against their antibacterial and antifungal stains. Based on the observed mm of zone of inhibition values the effect of antibacterial and antifungal activities of all sulphonamides are studied.
Materials and Methods
Experimental
Sigma-Aldrich, Alfa Aesar and E Merck chemical company chemicals used in this investigation. Mettler FP51 melting point apparatus was used for the determination melting points of all sulfonamides. The OMNIC Fourier-transform spectrophotometer was employed for recording Infrared spectra. The proton and carbon-13 spectra of all sulfonamides were recorded in (Bruker AV400 & 500 NMR spectrometer in DMSO solvent using TMS as internal standard. Mass spectra were recorded on a SHIMADZU GC-MS2010 spectrometer. The Thermo Finnigan CHN analyzer was used for elemental analysis.
Experimental procedure for the synthesis of mesalazine sulphonamides
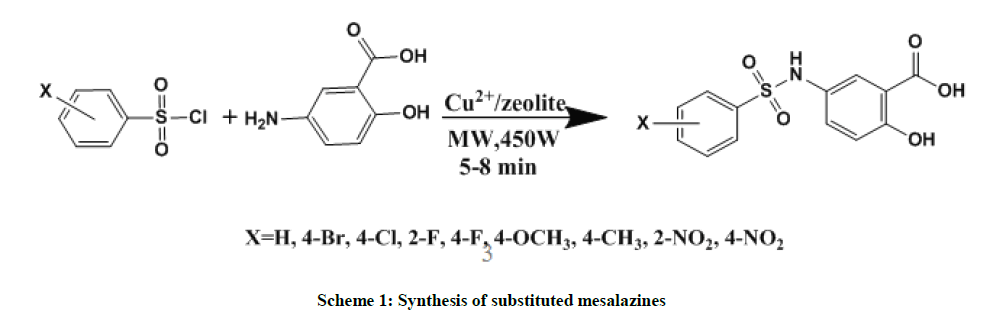
An equimolar quantities of substituted sulfonyl chlorides (1 mmol), mesalazine (1 mmol) and Cu2+/zeolite (80 mg) clay catalyst subjected to microwave irradiation for 5-8 min in a microwave oven (Scheme 1) at 450 W (Samsung Grill, GW73BD Microwave oven, 230 V A/c, 50 Hz, 2450 Hz, 100-750 W (IEC-705)) [8]. During the reaction about 0.1 ml of triethylamine was added to neutralize the formation of hydrochloride. The resulting product was washed with n-hexane and separated the catalyst using methanol by filtration and dried. The analytical and spectroscopic data of synthesized mesalazine sulfonamides were presented in Tables 1 and 2.
| Entry | X | M.F | M.W | Yield (%) | Time (min) | m.p (°C) | C (%) Obd. (calcd.) |
H (%) Obd. (calcd.) |
N (%) Obd. (calcd.) |
Mass (m/z) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | C13H11NO5S | 293.3 | 88 | 5.3 | 276 | 53.26 (53.24) |
3.76 (3.78) |
4.72 (4.78) |
293 [M+] |
| 2 | 4-Br | C13H10BrNO5S | 372.19 | 90 | 5 | 305 | 41.99 (41.95) |
2.69 (2.71) |
3.71 (3.76) |
370 [M+], 372 [M2+] |
| 3 | 4-Cl | C13H10ClNO5S | 327.74 | 90 | 5 | 310 | 47.68 (47.64) |
2.99 (3.08) |
4.18 (4.27) |
327 [M+], 329 [M2+] |
| 4 | 2-F | C13H10FNO5S | 311.29 | 86 | 7 | 277-281 | 50.20 (50.16) |
3.18 (3.24) |
4.46 (4.50) |
311 [M+], 313 [M2+] |
| 5 | 4-F | C13H10FNO5S | 311.29 | 88 | 5 | 291-293 | 50.14 (50.16) |
3.20 (3.24) |
4.45 (4.50) |
311 [M+], 313 [M2+] |
| 6 | 4-OCH3 | C14H13NO6S | 323.32 | 85 | 8 | 301 | 52.08 (52.01) |
4.01 (4.05) |
4.29 (4.33) |
323 [M+] |
| 7 | 4-CH3 | C14H13NO5S | 307.32 | 85 | 8 | 282 | 54.68 (54.71) |
4.22 (4.26) |
4.49 (4.56) |
307.05 [M+] |
| 8 | 2-NO2 | C13H10N2O7S | 338.29 | 86 | 7 | 314 | 46.18 (46.16) |
2.92 (2.98) |
8.26 (8.28) |
338.02 [M+] |
| 9 | 4-NO2 | C13H10N2O7S | 338.29 | 90 | 5 | 320 | 46.15 (46.16) |
2.96 (2.98) |
8.25 (8.28) |
338.02 [M+] |
Table 1: The Physical constants, analytical and mass spectral data of mesalazine sulfonamide compounds
| Entry | X | Infrared bands (n, cm-1) | Chemical shifts (δ, ppm) | |||||
|---|---|---|---|---|---|---|---|---|
| N-H | S=Oasy | S=Osym | C=O | 1H | 13C | |||
| NH | COOH | C-OH | ||||||
| 1 | H | 3262.4 | 1311.9 | 1158 | 1695.9 | 10.832 | 172.4 | 159.64 |
| 2 | 4-Br | 3263.2 | 1332.8 | 1156 | 1670.8 | 10.853 | 172.36 | 159.83 |
| 3 | 4-Cl | 3262.5 | 1333.8 | 1157.3 | 1670.8 | 10.077 | 171.06 | 158.71 |
| 4 | 2-F | 3264.4 | 1321.2 | 1168.7 | 1694.1 | 10.364 | 171.58 | 158.1 |
| 5 | 4-F | 3264.5 | 1325.7 | 1161.5 | 1670.8 | 10.29 | 171.53 | 159.15 |
| 6 | 4-OCH3 | 3250.1 | 1333.9 | 1158 | 1677.3 | 9.893 | 171.67 | 158.69 |
| 7 | 4-CH3 | 3262.8 | 1333.3 | 1157.3 | 1669.9 | 10.33 | 171.65 | 158.74 |
| 8 | 2-NO2 | 3293.2 | 1322.8 | 1175.9 | 1665.6 | 10.446 | 171.53 | 159.35 |
| 9 | 4-NO2 | 3263.6 | 1311.9 | 1159.5 | 1670.8 | 10.399 | 171.5 | 159.21 |
Table 2: The spectroscopic data of substituted mesalazine sulphonamides
Results and Discussion
Antibacterial activity of sulphonamides by disk-diffusion method
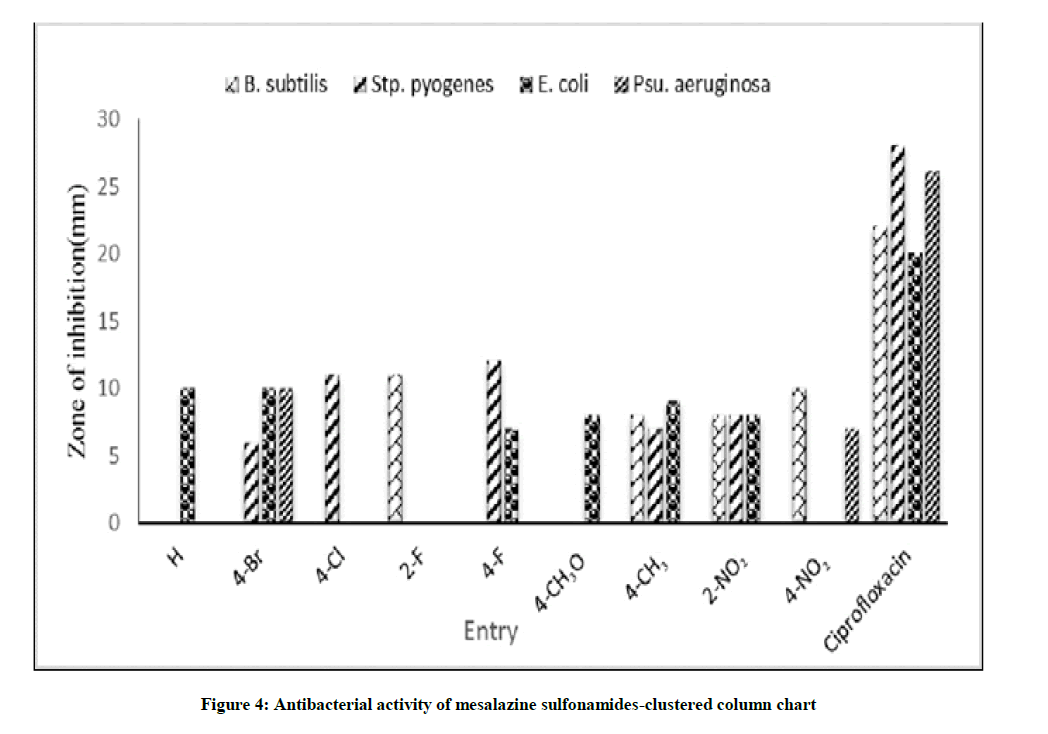
Antibacterial activities of all synthesized mesalazine sulfonamides were determined using standard well known Bauer-Kirby disc diffusion method. In this study the authors have chosen the Gram-positive bacterial strains such as B. subtilis and S. pyogenes and the Gram-negative bacterial strains such as E. coli, P.aeruginosa. The observed zone of inhibition of these compounds against their strains is shown in Table 3. The diameters (mm) of zone of inhibition of bacterial species are shown in Figure 1 and the correlated clustered column chart was shown in Figure 4. The antibacterial effect of mesalazine sulfonamides is shown in Figure 5 [9]. A satisfactory antibacterial activity showed B. subtilis, S. pyogenes antibacterial species when compared to standard ciprofloxacin drugs. The sulfonamide possess 2-F, 4-NO2¬ substituents have moderate activity against B. subtilis. The 4-Cl and 4-F substituted sulfonamides have shown moderate antibacterial activity against S. pyogenes. The parent and 4-Br substituted sulfonamides were shown moderate activity against E. coli. The 4-Br substituted sulfonamide only show the moderate activity against P. aeruginosa [10-12].
| Entry | X | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Gram positive Bacteria | Gram negative Bacteria | ||||
| Bacillus subtilis | Streptococcus pyogenes | Escherichia coli | Pseudomonas aeruginosa | ||
| 1 | H | - | - | 10 | - |
| 2 | 4-Br | - | 6 | 10 | 10 |
| 3 | 4-Cl | - | 11 | - | - |
| 4 | 2-F | 11 | - | - | - |
| 5 | 4-F | - | 12 | 7 | - |
| 6 | 4-OCH3 | - | - | 8 | - |
| 7 | 4-CH3 | 8 | 7 | 9 | - |
| 8 | 2-NO2 | 8 | 8 | 8 | - |
| 9 | 4-NO2 | 10 | - | - | 7 |
| Standard | Ciprofloxacin | 22 | 28 | 20 | 26 |
| Control | DMSO | - | - | - | - |
Table 3: The antibacterial screening effect of synthesized mesalazine sulfonamide derivatives
Antifungal activity of sulphonamides by disk-diffusion method
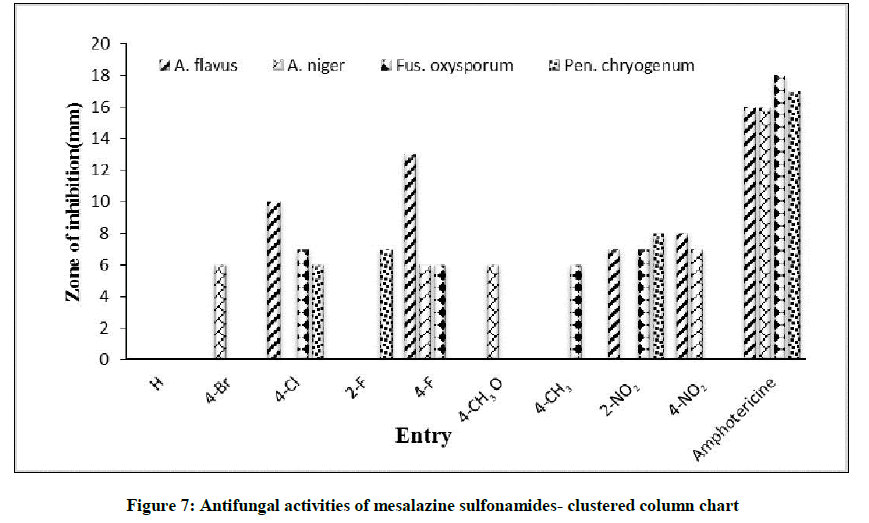
Antifungal activities of all synthesized mesalazine sulfonamides were determined using standard well known Bauer-Kirby disc diffusion method. The test organisms were sub cultured using PDA medium. In this study, the authors had chosen four fungal strains such as Aspergillus flavus, Aspergillu niger, Fusarium oxysporum and Penicillium chrysogenum. The observed mm of zone of inhibition of antifungal activities values are presented in Table 4. The anti-fungal activities of substituted mesalazine sulfonamides by means of petri dishes are shown in Figure 6. The statistical comparison data-cluster column chart of fungal activities is illustrated in Figure 7. The sulfonamides possess 4-Cl and 4-F have been shown excellent activity against Aspergillus flavus. The 4-NO2 substituted sulfonamides have shown moderate antifungal activity against Aspergillus nigar strain. The sulfonamides containing 4-Cl, 2-F, 4-F, 4-CH3, 2-NO2 where shown less moderate antifungal activities against F. oxysporum and P. chrysogenum strains [13-15].
| Entry | X | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Aspergillus flavus | Aspergillus niger | Fusarium oxysporum | Penicillium chrysogenum | ||
| 1 | H | - | - | - | |
| 2 | 4-Br | - | 6 | - | - |
| 3 | 4-Cl | 10 | - | 7 | 6 |
| 4 | 2-F | - | - | - | 7 |
| 5 | 4-F | 13 | 6 | 6 | - |
| 6 | 4-OCH3 | - | 6 | - | - |
| 7 | 4-CH3 | - | - | 6 | - |
| 8 | 2-NO2 | 7 | - | 7 | 8 |
| 9 | 4-NO2 | 8 | 7 | - | - |
| Standard | Amphotericin-B | 16 | 16 | 18 | 17 |
| Control | DMSO | - | - | - | - |
Table 4: The antifungal screening effect of mesalazine sulfonamides
Conclusion
The synthesized sulfonamides were characterized by their physical constants, spectral data. The antibacterial activities of all synthesized sulfonamides have been studied using Bauer-Kirby method. The 2-F, 4-NO2, 4-Br/4-Cl, 4-F, parent and 4-Br/4-Cl substituted sulfonamides have shown modulate antibacterial activity against their bacterial strains. The sulfonamide possess 4-Cl and 4-F substituents have shown excellent antifungal activities against Aspergillus flavus fungal strain. The 4-Cl, 4-Br, 4-F, 4-OCH3, 2-F, 2-NO2, 4-NO2, 4-CH3, substituted sulfonamides shown less moderate antifungal activities against Aspergillus niger, F. oxysporum, P. chrysogenum fungal strains.
Acknowledgement
Authors thank to DST-NMR facility, Department of Chemistry, Annamalai University, Annamalainagar-608002 for recording NMR spectra of all sulphonamides.
References
- T.N. Raju, Lancet., 1999,353, 681.
- A. Kołaczek, I. Fusiarz J. Lawecka, D. Branowska, Chemik., 2014, 68, 620,
- T. Narasaiah, D.S. Rao, K.V. Ramana, S. Adam, C.N. Raju, Der Pharma Chemica, 2012, 4, 1582.
- T. Claudiu, A.C. Supuran, A. Scozzafava, Med. Res. Rev., 2003, 23, 535.
- C.N. Igwe, U.C. Okoro, Org. Chem. International., 2014, 5, 419.
- H. Yoshino, N. Ueda, J. Niijima, H. Sugumi, Y. Kotake, N. Koyanagi, K. Yoshimatsu, M. Asada, T. Watanabe, J. Med. Chem., 1992, 35, 2496..
- C.M. Rothschild, M.T. Hines, B. Breuhaus, J. Gay, D.C. Sellon, J. Vet. Intern. Med., 2004, 18, 373.
- C. Temperini, A. Cecchi, A. Scozzafava, C.T. Supuran, Org. Biomol. Chem., 2008, 6, 2499.
- A.W. Bauer, W.M. Kiruby, J.C. Sherris, M. Truck, Am. J. Clin. Pathol., 1966, 45, 493.
- S. Dineshkumar, G. Thirunarayanan, P. Mayavel, I. Muthuvel, Ovidius Univ. Annals Chem., 2016, 27(1) 22.
- R. Arulkumaran, R. Sundararajan, S. Vijayakumar, S. P. Sakthinathan, R. Suresh, D. Kamalakkannan, K. Ranganathan, G. Vanangamudi, G. Thirunarayanan, J. Saudi Chem. Soc., 2016, 20, S122.
- G. Thirunarayanan, G. Vanangamudi, Arab J. Chem., 2016, 9, S269.
- G. Thirunarayanan, Arabian J. Chem., 2017, 10(S1), S636.
- V. Usha, V. Thangaraj and G. Thirunarayanan, Indian J. Chem., 2017, 56B(10), 1094.
- D. Kamalakkannan, G. Vanangamudi, G. Thirunarayanan, Der Pharma Chemica, 2017, 9(12), 34.