Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 4
Antifungal Activity of Condensed Benzothiazole and Its Derivatives
Anil B Chidrawar*Anil B Chidrawar, P.G. Department of Chemistry, Degloor College, Degloor-431717, S.R.T.M.U, Nanded, Maharashtra, India, Email: anilchidrawar74@gmail.com
Abstract
A simple and efficient method have been used for the synthesis of 2-substituted derivatives of 3-cyano-4,14-diimino-2-methylthio-10-nitropyrimido [2,3-b] pyrazolo [3,4-e] pyrimido [2,3-b] [1,3] benzothiazole have been prepared through one Step Multicomponent reaction by heating a mixture of 3-amino-4-imino-8-nitro-2H-pyrazolo[3,4-e]pyrimido[2,3-b][1,3]benzothiazole and bis (methylthio) methylene malononitrile independently with aromatic amines/phenols/heterylamines/compounds containing active methylene group respectively in the presence of dimethyl formamide and catalytic amount of anhydrous potassium carbonate. All these newly synthesized compounds were screened for anticancer activity.
Keywords
Bismethylthiomethylene, Malononitrile, Dimethyl formamide, Potassium carbonate
Introduction
The antifungal activity [1-14] of synthesized compounds can be determined by screening them against the fungal species using microbial method (assay). The basic principle of microbial assay lies in the comparison of the inhibition of growth of fungi by known concentration of test compounds with that of known concentration of standard antifungal agent (fluconazole) having known activity. Generally, two types of methods were used for determination of antifungal activity.
A survey of literature made it evident that very little work has been carried out on the synthesis of fused pyrimido benzothiazoles possessing three–four rings [15,16] which exhibit a wide spectrum of activities like anti-tumor [17], phosphodiesterase inhibition [18], antiallergic [19], anti-inflammatory [20] and anti-parkinsonism [21].
Agar plug method
The fungicidal effect of the compound can be assessed by the inhibition of mycelia growth of the fungus and is observed as a zone of inhibition near the disc or the wells.
Reagents
Potato dextrose agar medium
The commercially available (HiMedia) potato dextrose agar medium (39 g) was suspended in 1000 ml of distilled water. The medium was dissolved completely by boiling and was then autoclaved at 15 lbs pressure (121°C) for 15 min. Fluconazole (standard antifungal agent).
Procedure
Potato dextrose agar medium was prepared and poured on to the petriplates. A fungal plug was placed in the center of the plate. Sterile discs immersed in the solution of newly synthesized compounds were also placed in the plates. Fluconazole was used as antifungal control. The antifungal effect was seen as crescent shaped zones of inhibition.
Spore germination assay
Lacto phenol cotton blue stains the fungal cytoplasm and provides a light blue background, against which the walls of the hyphae can readily be seen. It contains four constituents: phenol which serves as a fungicide, lactic acid as cleaning agent, cotton blue to stain the cytoplasm of the fungus and glycerol to give a semi-permeable preparation.
Reagents
Lacto phenol cotton blue stain, phenol crystals (20 g), cotton blue (0.05 g), lactic acid (20 ml), glycerol (20 ml), distilled water (20 ml). The stain was prepared by dissolving the chemicals with gentle heating for complete dissolution.
Procedure
Aliquots of spore were prepared by mixing loopfull of fungal spores in sterile distilled water. 25 μl of spore suspension was added to 10 μl of the tested compound solution and placed in separate glass slides. Slides with 25 μl of spore suspension alone served as the controls. Slides were then incubated in moist chamber at 25°C for 24 h. Each slide was fixed in lacto phenol cotton blue stain. The mold was mixed gently with the stain using two teasing needles. A cover slip was placed on the preparation and examined under the phase contrast microscope (Kozo XJS500T, Japan) for spore germination.
Experimental section
All melting points were determined in open capillary tube and were uncorrected. IR spectra were recorded with potassium bromide pellets technique, 1H NMR spectra were recorded on AVANCE 300 MHz Spectrometer in DMSO using TMS as internal standard. Mass spectra were recorded on a FT VG-7070 H Mass Spectrometer using EI technique at 70 eV. All the reactions were monitored by thin layer chromatography (TLC).
Material and Methods
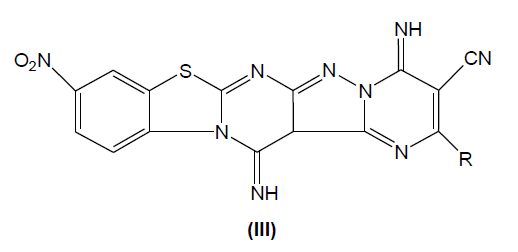
Synthesis of 2-Substituted derivative of 3-cyano-4,14-diimino-2-methylthio-10-nitro pyrimido[2,3-b]pyrazolo [3,4-e]pyrimido[2,3- b][1,3]benzothiazole (III)
A mixture of 3-amino-4-imino-8-nitro-2H-pyrazolo [3,4-e] pyrimido [2,3-b] [1,3] benzothiazole (0.301 g, 0.001 mol) and bis-methylthio methylene malononitrile (0.170 g, 0.001 mol) was refluxed in the presence of dimethyl formamide (5 ml) and a pinch of anhydrous potassium carbonate (0.2 g) was refluxed independently with one mole equivalent of aryl amines/phenols/heteryl amines and compounds containing active methylene group for 6 h. The progress of reaction was monitored on TLC. After completion of reaction, the reaction mixture was cooled to room temperature and poured on ice cold water. The separated solid product was filtered, washed with water and recrystallized from ethanol to give respective products.
Method for antifungal activity
Antifungal activity by disc diffusion method
In this method the sensitivity of synthesized compounds is measured by determining the zone of inhibition after placing paper disc dipped in solution of compounds. These results were compared with the zone of inhibition produced after placing disc dipped in solution of standard antibiotic (Figure 1).
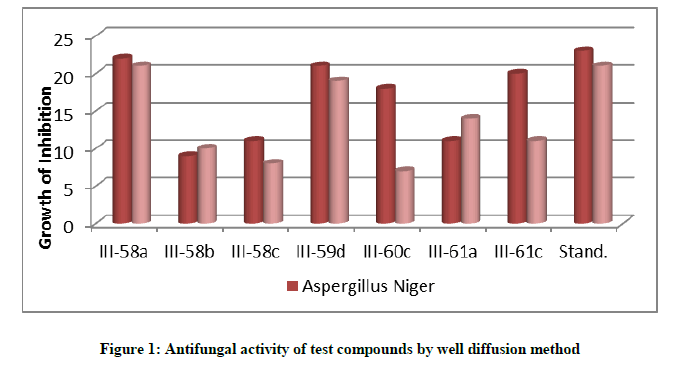
Antifungal activity by well diffusion method
The in vitro antifungal activity by agar well diffusion method was standardized using Fluconazole. This method is based on diffusion of antifungal component from reservoir hole to the surrounding inoculated Potato dextrose agar medium, so that the growth of fungus is inhibited as zone around the hole. Two fungi were selected viz. Aspergillus niger and Penicillium sp.
Organisms selected for antifungal activity
The organisms selected for antifungal activity are A. niger and Penicillium sp species. Antifungal activity by well diffusion method of 3-cyano-4,14-diimino-2-methylthio-10-nitropyrimido[2,3-b]pyrazolo[3,4-e]pyrimido[2,3-b][1,3]benzothiazole (III) and its 2-substituted derivatives (Figure 2).
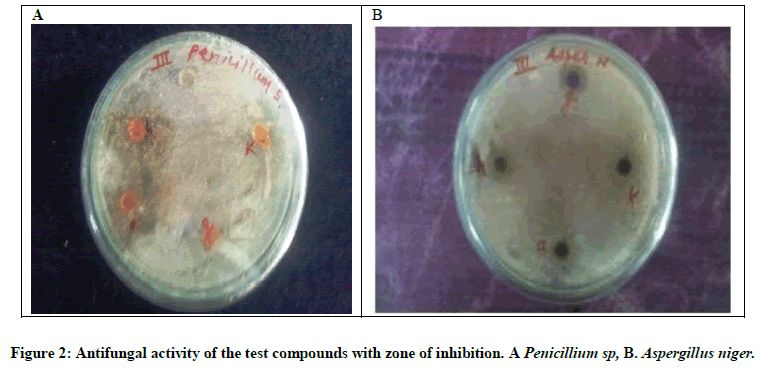
Organisms selected for antifungal activity
The organisms selected for antifungal activity are A. niger and Penicillium sp species. Antifungal activity by well diffusion method of 3-cyano-4,14-diimino-2-methylthio-10-nitropyrimido[2,3-b]pyrazolo[3,4-e]pyrimido[2,3-b][1,3]benzothiazole (III) and its 2-substituted derivatives (Figure 2).
Conclusion
Two moieties are fused and screened for antifungal studies they showed a broad spectrum of antifungal activity. They showed good activity against Penicillium sp. and A. niger species. 3-cyano-4,14-diimino-2-methylthio-10-nitropyrimido [2,3-b] pyrazolo [3,4-e] pyrimido [2,3-b] [1,3] benzothiazole (III) and its 2-substituted derivatives are responsible for antifungal activity, but it is interesting to note that benzothiazole moieties when fused with other moieties showed a broad spectrum antifungal activity. Hence in search of new generation of antibiotics it may be worthwhile to explore the possibility in this area by fusing different moieties and increase potency.
Acknowledgements
The authors are thankful to the Department of Microbiology, Vai. Dhunda Maharaj College, Degloor an affiliated College to Swami Ramanand Teerth Marathwada University, Nanded for providing laboratory facilities.
References
[1] I. Hutchinson, M. Chua, H.I. Browne, V. Trapani, T.D. Bradshaw, A.D. Westwell and M.F.G. Stevens, J. Med.Chem., 2001, 44, 1446.
[2] G.M. Catriona, G. Wells, J.P. Crochard, E.L. Stone, T.D. Bradshaw, M.F.G. Stevens, A.D. Westwell, J. Med. Chem., 2005.
[3] G. Sirockin, S. Cullimore, Mc Graw Hill Publishing Co. Ltd., Londan, 1969.
[4] V. Lorian, William and Wilkins, Baltimore, 1986.
[5] M.J. Pleczer, R.D. Reid, E.C.S. Chan, Tata Mc Graw, Hill Publishing Co. Ltd., London, 1978.
[6] Indian Pharmacopoeia, Vol.â??I and II, Government of Indian publication, 1996.
[7] A.W. Bauer, W.M.M. Kirby, J.C. Sherris, Am. J. Clin. Pathol., 1966, 136, 493.
[8] N.E. Quiroga, R.A. Sampietro, A.M. Valtuone. J. Ethano. Pharmacol., 2001, 74, 89.
[9] J. Meletiadis, J.W. Mouton, J. Meis, B.A. Bouman, P.E. Verweij, N. Eurofung., J. Clinic. Microbiol., 2002, 40, 2876.
[10] R. Dabur, A.K. Chhillar, V. Yadav, K. Kamal, P. Gupta, G.L. Sharma, Fitoterapia., 2004, 75(3), 389.
[11] M.D. Maji, S. Chattopadhyay, P. Kumar, B.C. Sarat, Archies. Phytopatholo. Plant. Prot., 2005, 38, 157.
[12] G. Schmourlo, M.R. Filho, S.C. Alvino, S.S. Costa, J. Ethano. Pharmacol., 2005, 96, 563.
[13] R. Dabur, A.K. Chhillar, V. Yadav, K. Kamal, P. Gupta, G.L. Sharma., J. Med. Micro., 2005, 54, 549.
[14] S. Sahoo, P.K. Panda, S. Tripathy, S.K. Nayak, S.K. Dash., Indian. Drugs., 2007, 44(5), 352.
[15] R. Gompper, W. Toepfl, Chem. Ber., 1962, 2871.
[16] R.J. Alaimo, J. Het. Chem., 1973, 10(5), 769-72.
[17] H.L. Peter, U.S. Patent, 3704303, Chem. Abstr., 1972, 78, 43513.
[18] R.R. Connston, D.L. Temple, J.P. Yevich, Ger. Patent., 2918085, Chem. Abstr., 1979, 92, 1639939.
[19] S. Victro, Eur. Patent, 2180, Chem. Abstr., 1981, 94, 2089005.
[20] R.A. Glennon, J.J. Gaines, M.E. Rogers, J. Med. Chem., 1981, 24(6), 766-769.
[21] J.J. Wade, C.B. Toso, C.J. Matsm, V.L. Stelzer, J. Med. Chem., 1983, 26, 608.



