Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 2
Anti-Corrosive Behavior of Senna Aqueous Extract to Aluminum in Alkaline Solutions
Ehteram A Noor, Aisha H Al-Moubaraki and Reem M Alghanmi
Abstract
Electrochemical measurements (EIS and PDP) were used to evaluate the anti-corrosive behaviour of SAE on Al in 0.25 M NaOH solutions. The results showed that the inhibition efficiency increases with increasing SAE concentration. PDP measurements revealed that SAE acts as a mixed type inhibitor against the corrosion of Al in the studied solution. EIS measurements indicated the formation of a compact adsorbed layer by inhibitor species on Al surface. Different adsorption isotherm models were tested and paramount fitting was obtained by Langmuir isotherm model. The analysis of FTIR spectra confirmed the formation of a strong bond between inhibitor species and Al surface.
Keywords
Aluminum, Corrosion, Senna, Polarization, Impedance, Inhibition, FTIR
Introduction
In the past, the term "oxidation" was consistently used in place of the term "corrosion". Nevertheless, the latter is the right term because corrosion is also an electrochemical reaction, during which the metal is oxidized, and indicates its conversion into an oxide, i.e. the form in which it existed in the natural ores [1]. Aluminum is actually a very effective reactive metal, and one of its important features is the tendency to undergo oxidation relatively quickly. The resultant aluminum oxide covers the metal surface, and protects the interior aluminum from any further reaction [2]. However, if anything corrodes this layer, then the attack becomes rapid until the aluminum is consumed [3]. The dissolution rate of aluminum oxide depends on the pH value. It is higher at acidic and alkaline pH values, which reveals the amphoteric properties of aluminum oxide [1]. In general, aluminum surfaces corrode in higher alkaline solutions by forming soluble species (AlO2 –) and generating hydrogen molecules (H2), according to the following redox reaction [4]:
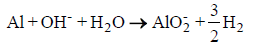 (1)
(1)
One strategy of reducing aluminum corrosion is the use of inhibitors that decrease the dissolution rate of the corroded metal to a recommended level with little environmental impact [5]. The research field of corrosion inhibitors is going through extraordinary changes the ecological concern point of view. Ecological rules require corrosion inhibitors to be green, environmentally friendly and safe [5]. Some current studies have used of green inhibitors acquired from plant sources, to control the deterioration of aluminum in alkaline solutions. These inhibitors include Gum Arabic [6], Sansevieria trifasciata extract [7], Damsissa (Ambrosia maritime L.) extract [8], Gossipium hirsutum L. extracts [9], Hibiscus sabdariffa leaves extract [4], Azwain (Trachyspermum copticum) seed extract [10] and Euphorbia hirta and Dialium guineense leave extracts [11], Senna auriculata leaves extract [12] and Neolamarkia cadamba bark extact [13]. It is worth noting that most of the literature agree well that the inhibition properties of natural inhibitors may be due to the presence of wide variety of organic compounds containing O, S or N/or a combination of the atoms in their extracts. Several studies have reported that similar organic compounds can be used as inhibitors for aluminum corrosion in alkaline solutions [14-20]. Generally, the core mechanism of inhibition is no different, whether using pure organic compounds or natural products, which is built mainly on physical adsorption and/or chemical adsorption for the inhibitor species on the metal surface. The resultant adsorbed layer reduces the surface area that is available for the attack of aggressive ions in the test solution. Valuable information on adsorption can be derived from the adsorption isotherms. Langmiur [10,21], Temkin [22,23], Frumkin [24,25], Freundlich [7,26] and Flory-Huggins [27] adsorption isotherms are the most common models used to fit the adsorption data of various corrosion inhibitors. Recently, the Dubinin-Radushkevich adsorption isotherm was used successfully to distinguish the type of inhibitor adsorption, whether chemical or physical adsorption [4,28].
Senna leaves are one of the medicinal herbs, which have been used for a long time in the Eastern and Western countries for the treatment of constipation. It is commercially available and consists of dried leaflets of Alexandria senna (Cassia acutifolia Delile) or Tinnevelly senna (Cassia angustifoliaVahl) belonging to the plant family Leguminosae [29,30]. The active constituents of Senna are known to be sennosides A, B, C and D, while 1,8-dihydroxyanthraquinone derivatives such as chrysophanol, aloe-emodine, rhein and their glycosides are also present [31].This work is aimed (i) to study the effect of Senna Aqueous Extract (SAE) on the Al corrosion rate in 0.25 M NaOH using electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP) measurements, (ii) to analyze the adsorption data using various adsorption isotherm models, and (iii) to analyze the protective layer formed on Al by using Fourier transform infrared (FTIR) spectroscopy.
Experimental
Inhibitor preparation
SAE was prepared as follows: 25 g of dried senna leaves (Figure 1) were crushed and mixed with 250 mL of deionized water in suitable round bottom flask. The mixture was heated for 1 h on a boiling water bath. After filtration, the water was evaporated from the extract in an oven at controlled temperature (50-60ºC) to yield a dark brown crystalline precipitate (Figure 2b). Accordingly, the concentration of SAE was expressed as w/v.
Working electrode preparation
The chemical composition of the commercially pure aluminum used in the present study was (wt%): 0.002% Cu, 0.003% Zn, 0.100% Pb, 0.050% Co, 0.011% Ni, 0.179% Fe, 0.082% Cr and 99.574% Al. A rod of 5 cm in length and 1 cm in diameter was used as the working electrode, after inserting into a Teflon tube just larger than the specimen and fixed with an adhesive. In this case, the cross-sectional area exposed to the solution was 0.785 cm2. Surface preparation of the specimens was carried out using SiC abrasive papers ranging from 80 to 1000 grit. Afterwards, the abraded aluminum surface was washed with deionized water, degreased with acetone and finally dried at room temperature before immersion in the tested solution.
Test solutions preparation
1 M NaOH stock solution was prepared by dissolving the appropriate amount of NaOH (analytical grade) in 2 L of deionized water. Alkaline solutions for each test (0.25 M) were newly prepared by dilution, either in the absence or in the presence of a certain concentration of inhibitor. The inhibitor concentrations (0.25-3.5 g.L-1) were prepared by adding an appropriate amount of SAE to the aggressive solution. The volume of test solution for each experiment was 100 mL. All test solutions were stagnant and not deaerated before use and maintained at 30ºC.
Procedures
Electrochemical methods
Electrochemical Impedance Spectroscopy (EIS) and potentiodynamic polarization (PDP) were used to assess the Al corrosion rates, in the absence and presence of inhibitor using ACM Gill AC Potentiostat/Galvanostat model 655. These techniques enable the scientist to determine corrosion rate with high sensitivity, assess rate controlling mechanisms, and in some cases make life predictions [32]. EIS and PDP were carried out using a three-electrode cell design consisting of Al as the working electrode, a platinum wire as auxiliary electrode, and Ag/AgCl/KClsat electrode was used as a reference. EIS measurements were carried out in the frequency range of 30 kHz–0.1 Hz, at the steady state potential, by applying 30 mV sine wave ac voltage. EIS spectra were plotted in the Nyquist and Bode formats. For PDP measurements, the potential was scanned at a scan rate of 1 mV s-1 from the cathodic (-1,700 mV) to anodic (-1,300 mV) potentials. Electrochemical parameters were extracted using ACM software analysis version 4 based on Randle equivalent circuit for EIS spectra and Tafel ruler extrapolation method for PDP curves.
FTIR method
FTIR spectra were recorded in a Perkin- Elmer spectrophotometer model Frontier (USA), which extended from 400 to 4000 cm-1 using the KBr disk technique. The first sample of FTIR characterization is the SAE powder, which mixed with KBr and compressed as a disk. The second sample was the skinny film adsorbed on asteel surface after immersion for 72 h in 0.25 M NaOH solution containing 1.5 g.L-1 SAE, which was first cleaned, well dried, rubbed with a small amount of KBr and compressed as a desk.
General Remarks
• SAE was found to be an effective inhibitor against the corrosion of Al in an alkaline solution.
• The inhibition efficiency increases with increase in SAE concentration.
• PDP measurements revealed that SAE acts as a mixed type inhibitor for the corrosion of pure Al in 0.25 M NaOH.
• EIS measurements indicates the formation of an adsorbed film on the Al surface from the alkaline inhibited solution.
• Different adsorption isotherm models were tested and paramount fitting was obtained by the Langmuir isotherm model.
• Based on the negative values of ΔGads obtained from both EIS and PDP measurements, SAE species were adsorbed spontaneously on Al surface from 0.25 M NaOH.
• Good agreement between results obtained from EIS and PDP measurements was observed.
• Analysis of FTIR spectra confirms the adsorption of SAE species on Al surface.
References
[1] C. Vargel, M. Jacques, M.P. Schmidt, Elsevier, Netherland, 2004.
[2] http://www.cmiengineer.com/whitepapers/aluminum_corrosion.pdf
[3] R.B. Spacht, J. Chem. Educ., 1946, 23(5), 253.
[4] E.A. Noor, J. Appl. Electrochem., 2009, 39, 1465.
[5] V.S. Sastri, Winston Revie, Series Editor, 2011.
[6] S.A. Umoren, I.B. Obot, E.E. Ebenso, P.C. Okafor, O. Ogbobe, Anti-Corros. Method M., 2006, 53(5), 77.
[7] E.E. Oguzie, Corros. Sci., 2007, 49(3), 1527.
[8] A.M. Abdel-Gaber, E. Khamis, H. Abo-El Dahab, Sh. Adeel, Mater. Chem. Phys., 2008, 109 (2-3), 297.
[9] O.K. Abiola, J.O.E. Otaigbe, O.J. Kio, Corros. Sci., 2009, 51, 1879.
[10] A. Singh, M.A. Quraishi, Res. J. Recent. Sci., 2012, 1, 57.
[11] L.A. Nnanna, I.U. Anozie, C.S. Akoma, I.M. Mejeha, K.B. Okeoma, K.I. Mejeh, Amer. J. Mater. Sci., 2011, 1(2), 76.
[12] A. Sirajunnisa, M.I. Fazal Mohamed, A. Subramania, B.R. Venkatraman, IJSEAT, 2014, 2, 58.
[13] N. Chaubey, V.K. Singh, M.A. Quraishi, E.E. Ebenso, Int. J. Electrochem. Sci., 2015, 10, 504.
[14] B. Müller, Pigm. Resin Technol., 2002, 31(2), 84.
[15] B. Müller, Corros. Sci., 2004, 46, 159.
[16] D. Mercier, M.G. Barthés-Labrousse, Corros. Sci., 2009, 51, 339.
[17] S. Edrah, S.K. Hasan, J. Appl. Sci. Res., 2011, 6(8), 1045.
[18] H.N. Soliman, Corros. Sci., 2011, 53, 2994.
[19] M. Lashgari, Electrochim. Acta., 2011, 56, 3322.
[20] P.D.R. Kumari, J. Nayak, A.N. Shetty, J. Coat. Technol. Res., 2011, 8(6), 685.
[21] A.M. Al-Turkustani, S.T. Arab, L.S.S. Al-Qarni, J. Saudi Chem. Soc., 2011, 15, 73.
[22] S.A. Umoren, I.B. Obot, E.E. Ebenso, N. Obi-Egbedi, Portu. Electrochim. Acta., 2008, 26, 199.
[23] E.A. Noor, Mater. Chem. Phys., 2011, 131, 160.
[24] A. Chetouani, B. Hammouti, M. Benkaddour, Pigm. Resin Technol., 2004, 33(1), 26.
[25] L. Valek, S. Martinez, Mater. Lett., 2007, 61, 148.
[26] U.M. Eduok, S.A. Umoren, A.P. Udoh, Arab. J. Chem., 2012, 5, 325.
[27] N.O. Eddy, P.A.P. Mamza, Portu. Electrochim. Acta., 2009, 27(4), 443.
[28] M.M. Solomon, S.A. Umoren, I.I. Udosoro and A.P. Udoh, Corros. Sci., 2010, 52, 1317.
[29] United States pharmacopoeia 27. Rockville, MD.U.S pharmacopoeia convention: 1686, 2004.
[30] T.E. Wallis, Ed. Text book of pharmacognosy, 5th Edn., CBS publishers, New Delhi, 2004, 136-138.
[31] S. Hayashi, A. Yoshida, H. Tanaka, Y. Mitani, K. Yoshizawa, Chem. Pharm. Bull., 1980, 28, 406.
[32] G.S. Frankel, JASTM Int., 2008, 5(2), 1.
[33] Y. Yan, W. Li, L. Cai, B. Hou, Electrochim. Acta., 2008, 53(20), 5953.
[34] A.K. Satapathy, G. Gunasekaran, S.C. Sahoo, K. Amit, P.V. Rodrigues, Corros. Sci., 2009, 51, 2848.
[35] H.B. Shao, J.M. Wang, Z. Zhang, J.Q. Zhang, C.N. Cao, Corrosion (NACE)., 2001, 57(7), 577.
[36] A.A. Mazhar, S.T. Arab, E.A. Noor, Bull. Electrochem., 2001, 17(10), 449.
[37] K.F. Khaled, Electrochim. Acta, 2009, 54, 4345.
[38] A.Y. El-Etre, Corros. Sci., 2003, 45, 2485.
[39] S. Karahan, M. Yurdakoc, Y. Seki, K. Yurdakoc, J. Colloid. Interface. Sci., 2006, 293 (1), 36.
[40] S. Eid, M. Abdallah, E.M. Kamar, A.Y. El-Etre, J. Mater. Environ. Sci., 2015, 6(3), 892.
[41] http://www.mdidea.com/products/new/new04005.html



