Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 11
An Expedient Synthesis of Ethyl 5-Acetyl-1,2,4,4a,5,6-Hexahydro Benzo-[G][1,4]Oxazino[4,3-A][1,8]Naphthyridine-5-Carboxylate from 2-Cholro-3-Formyl Quinoline
Sathiyamoorthy S1, Jemima D1, Pitchai P1*, Makhanya TR2 and Gengan RM2
1PG and Research Department of Chemistry, Government Arts College (Auto), Kumbakonam 612002, Tamilnadu, India
2Department of Chemistry, Steve Biko Campus, Durban University of Technology, Durban 4000, South Africa
- *Corresponding Author:
- Pitchai P
PG and Research Department of Chemistry
Government Arts College (Auto)
Kumbakonam 612002, Tamilnadu, India
Abstract
The multi-component reactions of organic molecules are an important strategy for the synthesis of biologically active fused quinoline derivatives. This can be classified as a green strategy which organic chemists are reporting on a regular basis. The current study describes a strategy to synthesize ethyl 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate from 2-chloro-3-formyl quinoline, a readily available and cost effective substrate.
Keywords
2-Chloro-3-formyl quinoline, Ethylaceto acetate, Morpholine.
Introduction
Naphthyridine derivatives have received significant attention due to their exceptionally broad spectrum of biological activity. Nalidixic acid, for example, possesses strong antibacterial activity and is used mainly for the treatment of urinary tract infections with gram-negative pathogens [1]. Gemifloxacin possess both antimicrobial properties [2] whilst 1,8-naphthyridine derivatives have promising medicinal properties such as anti-HIV [3], anticancer [4], anti-inflammatory [5], antimalarial [6], antibacterial [7], antiprotozoals [8], antimycobacterial [9] and antiplatelet [10]. Moreover, it was recently found that 1,8-naphthyridine derivative vosaroxin (formerly SNS-595, AG-7352, AT-3639, or Voreloxin) was found to have potential anticancer activity; it is currently subjected to clinical development. This drug is reported to exert its action via topoisomerase II inhibition [11]. Topoisomerase II is one of the well-known targets for antitumor agents like doxorubicin, etoposide, ellipticine and amsacrine [12].
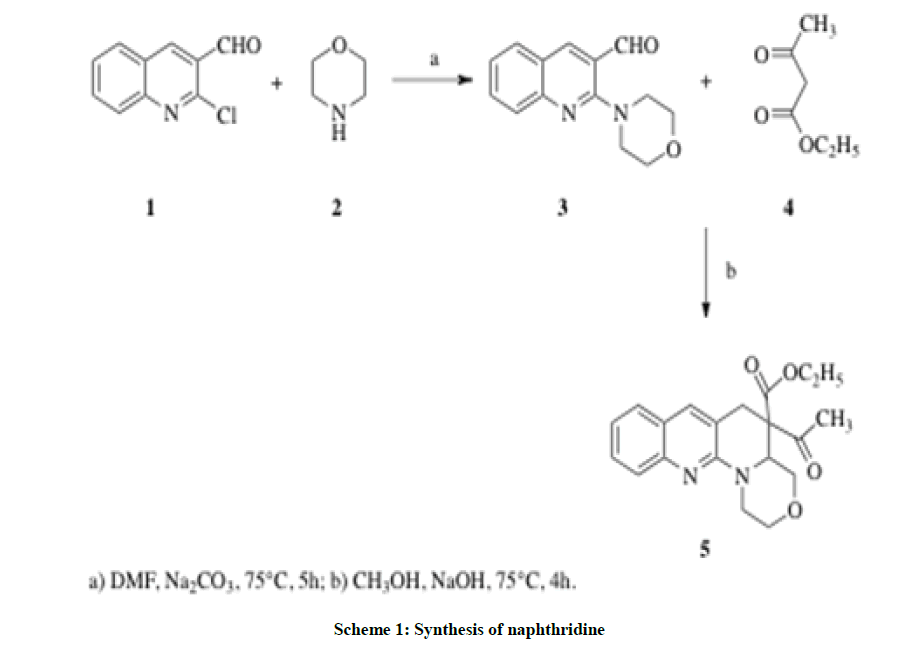
Various procedures are available for the synthesis of 1,8-naphthyridines, however, several disadvantages are associated with these methodologies including unsatisfactory yields, long conversion times and flammable organic solvents. In recent years, we have focused our efforts on the development of simple methods for the synthesis of naphthyridines derivatives [13-15]. Hence, now we report a useful general approach towards the formation of 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate 5.
Materials and Methods
General
Melting points are uncorrected. Infrared spectra were recorded on a Perkin-Elmer Paragon 1000 Fourier Transform Infrared(FTIR) spectrophotometer as potassium bromide discs unless otherwise indicated. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were obtained on a Brucker (400 MHz) instrument in CDCl3 solutions using tetramethylsilane as an internal standard. J Values are given in Hz. Mass spectra were obtained at the Vellore Institute of Technology, Vellore, Tamil Nadu, India. Column chromatography utilized Merck silica gel 60 and hexane and ethylacetate as eluants. All the basic chemicals were purchased from Merck (India) and Fluka (South Africa).
Synthesis of 2-chloroquinoline-3-carbaldehyde 1 [16]
Synthesis of 2-morpholinoquinoline-3-carbaldehyde 3
2-Chloroquinoline-3-carbaldehyde 1 was dissolved in 15 ml of N,N-dimethylformamide and morpholine was added drop-wise with a catalytic amount of sodium carbonate. The mixture was allowed to heat at 75ºC for 5 h. After complete conversion of the reaction, the mixture was poured into 1000 ml of ice-cold water. It was neutralized with dilute hydrochloric acid. The pale green colored precipitate was thereafter filtered and was purified through column chromatography in 100% petroleum ether.
Spectroscopic properties of 2-morpholinoquinoline-3-carbaldehyde 3
2-Chloroquinoline-3-carbaldehyde 1: 1.90 g (0.01 mol); morpholine 2: 0.87 ml; yield 1.815 g (75%) as a pale greenish solid, mp: 113ºC; IR( KBr, υmax, cm-1): 3439, 3043, 2850, 1689, 1593; 1H-NMR (400 MHz, DMSO-d6): δ (ppm)=11.381 (bs, 1H, -CHO), δ (ppm)=7.787 (s, 1H, H-4); δ (ppm)=7.694-7.674 (d, J = 8.0 Hz, 1H, H-5); δ (ppm)=(7.434-7.397, t, J = 7.6 Hz, 1H, H-7), δ (ppm)=7.245-7.225 (d, J = 8.0 Hz, 1H, H-8); δ (ppm)=(7.145-7.109, t, J = 7.1 Hz, 1H, H-7), δ (ppm)=7.245-7.225 (m, 8H, ali-oxazino-H); 13C-NMR (100 MHz, DMSO-d6): δ (ppm)=196.93, 166.34, 160.81, 139.10, 138.59, 133.08, 129.99, 128.15, 119.58, 112.82, 37.00, 30.42, 27.07, 20.48. GC-MS: 242(M+).
Synthesis of ethyl 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate 5
2-Morpholinoquinoline-3-carbaldehyde 3 was dissolved in 20 ml of methanol and ethyl acetoacetate was added: the reaction mixture was refluxed for 4 h at 75ºC. The mixture was allowed to cool to room temperature, poured into ice-cold water, filtered, dried and purified by column chromatography using ethyl acetate and petroleum ether (10: 90).
Spectroscopic properties of ethyl 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate 5
2-Morpholinoquinoline-3-carbaldehyde 3: 2.42 g (0.01 mol); ethyl acetoacetate: 1.30 ml; yield 2.30 g (65%) as a dark pink solid, mp: 98ºC; IR( KBr, υmax, cm-1): 2990, 1738, 1635, 1567; 1H-NMR (400 MHz, C DCl3): δ (ppm)=8.503 (s, 1H, H-7); δ (ppm)=7.705-7.853 (m, 3H, H-8, H-9 & H-11); δ (ppm)=7.420-7.385 (m, 1H, H-10); δ (ppm)=4.096-4.147 (m, 9H, H-1, H-2, H-3 & COO-CH2); δ (ppm)=3.488 (s, 2H, H-6), δ (ppm)=2.045 (s, 3H, Acetyl-CH3); δ (ppm)=1.233 (s, 3H, ethyl-CH3); 13C-NMR (100 MHz, CDCl3): δ (ppm)=189.97, 171.09, 158.82, 149.28, 143.20, 132.62, 129.24, 127.60. 124.84, 124.12, 122.04, 66.82, 60.36, 51.45, 21.01, 14.18; GC-MS: 354 (M+).
Results and Discussion
Different methods are reported in the literature to synthesize 2-chloro-3-formyl quinoline 1 scaffolds [16-20]. Our initial strategy targeted 2-morpholinoquinoline-3-carbaldehyde 3 and its conversion to the corresponding naphthyridine 5 by condensing with ethyl acetoacetate 4.
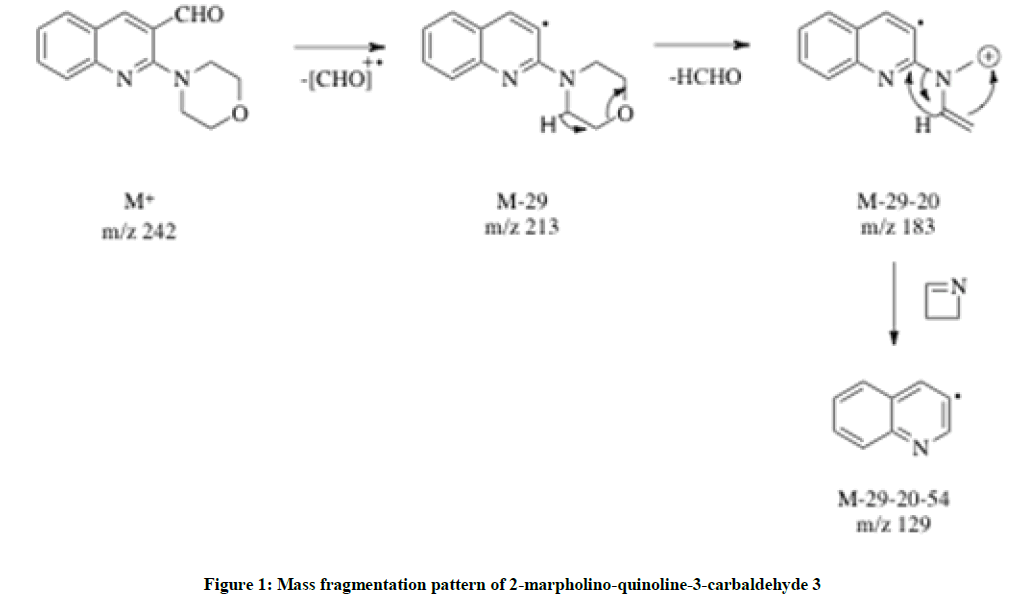
The synthesis of 2-chloro-3-formyl quinoline 1 was previously described as a two-step reaction using Otto Meth Kahn procedure via corresponding acetanilide [16]. Herein (Scheme 1), 2-chloro-3-formyl quinoline 1 was mixed with the equimolar amount of morpholine 2 in dimethylformamide; a catalytic amount of sodium carbonate was added and refluxed at 75°C or 5 h. The pale green mixture was then poured into ice cold water and the white precipitate was filtered, dried and purified through column chromatography in 100% petroleum ether elution. The yield was excellent and the solid melted at 113°C. The IR spectrum supported the identification of the structure by displaying the aldehydic C-H stretching at 2850 cm-1 and the C=O stretching at 1689 cm-1 and it implied that the aldehydic group remains unchanged. 1H-NMR spectra indicated two triplets recorded at δ 1.812-1.946 and δ 2.192-2.307 indicating the N-CH2- and the -CH2-O protons respectively. A broad singlet at δ 11.38 indicated the aldehydic C-H, which supported the substitution of oxazino ring at 2nd position of the quinoline. Carbon-13 Nuclear Magnetic Resonance (13C-NMR) showed four upfield values at δ 20.48, 27.07, 30.42, 37.00 and 39.34 are balanced for the oxazino ring and the aldehydic carbon peak was found at 196.93. Hence the structure was confirmed as 2-morpholinoquinoline-3-carbaldehyde 3 (Figure 1). The GC-MS spectrum further supported the compound 3 with its molecular ion peak found at m/z 242. The mass fragment pattern is as shown below.
Compound 3 was further treated with ethyl acetoacetate 4 in ethanolic sodium hydroxide at 75°C for 4 h. The reaction was monitored by thin layer chromatography. The solvent was distilled and the reaction mixture was transferred to a 1000 ml beaker containing ice-cold water and stirred. Then the solution was neutralized with 0.5 N HCl. The dark pink precipitate was filtered, dried and purified by column chromatography with a mixture of 70: 30 petroleum ether and ethyl acetate. The yield (60 %) was low and the solid melted at 98°C. The IR spectrum lacked the aldehydic C=O stretching at 1689 cm-1 but the appearance of two C=O stretching at 1738 cm-1 and 1635 cm-1 respectively for ketonic and carboxylic ester was visible. In 1H-NMR spectrum, a triplet for three protons at δ 1.226-1.277 and two proton quartet at δ 4.104-4.147 respectively for ethanoate CH3 and O-CH2 respectively were evident whereas protons of OC-CH3 was observed as a sharp singlet at δ 2.045 with three protons integration. Disappearance of the aldehydic proton and appearance of a singlet for two protons in the up field at δ 3.488 confirmed the cyclization through the insertion of a carbon of active methylene group of ethyl acetoacetate. 13C-NMR supported the insertion of active methylene group by slowing the characteristic carbonyl carbon chemical shifts at δ 171.09 and δ 189.97 for ketone and ester, respectively.
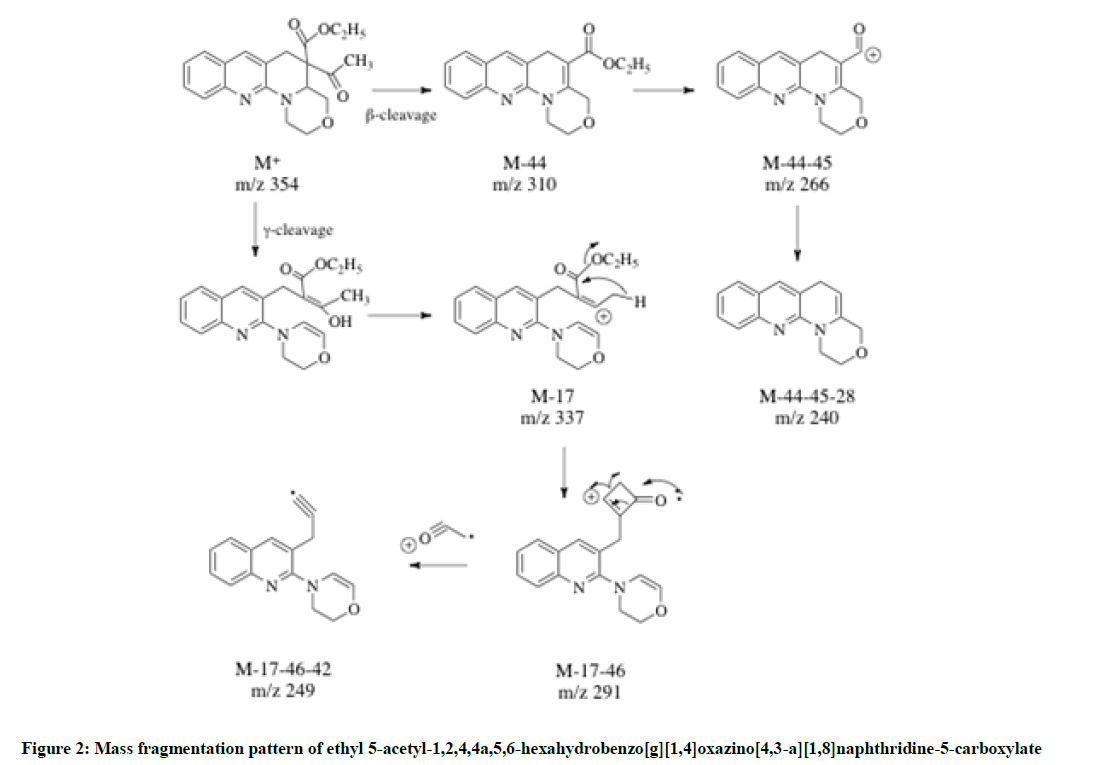
The molecular ion peak was recorded at m/z 355 which confirmed the formation of ethyl 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate 5 (Figure 2). The possible mass fragments are shown.
Conclusion
Herein we have reported a simple, convenient and high yielding procedure for the synthesis of ethyl 5-acetyl-1,2,4,4a,5,6-hexahydrobenzo[g][1,4]oxazino[4,3-a][1,8]naphthyridine-5-carboxylate a one-pot reaction. The simplicity of the methodology, ease of the product isolation and mild conditions indicates a green protocol.
Acknowledgement
The author thanks SRM University, Chennai and Vellore Institute of Technology for providing spectral data and S. Sathiyamoorthy thanks UGC, New Delhi for endorsement of the Rajiv Gandhi National Junior Research Fellowship.
References
- S. Jain, S. Arora, R. Saha, I.R. Kaur, Int. J. Med. Public Health., 2011, 1(4), 45.
- J. Yu-kan, Y.L. Hsu, Y.H. Chen, Bio. Med. Research inter., 2013, 159786, 11.
- S. Massari, D. Daelemans, M.L. Barreca, J. Med. Chem.,2010, 53, 641-648.
- A.A. Fadda, A.M. Defrawy, S.A. Habiby, Am. J. Org. Chem.,2012, 2, 87-96.
- G. Roma, G. Grossi, European. J. Med. Chem., 2008, 43, 1665-1680.
- S. Olepu, P.K. Suryadevara, Bioorg. Med. Chem. Lett.,2008, 18, 494-497.
- E. Laxminarayana, S. Karunakar, V. Shankar, M.T. Chary, Adv. Drug Delivery Rev.,2012, 2, 6-11.
- J.M. Quintela, C. Peinador, European J. Med. Chem., 2003, 38, 265-275.
- T. Aboul-Fadl, F.A.S. Bin-Jubair, O. Aboul-Wafa, European J. Med. Chem.,2010, 45, 4578-4586.
- P.L. Ferrarini, C. Mori, M. Badawneh, Farmaco.,2000, 55, 603-610.
- Y. Tsuzuki, K. Tomita, K. Shbamori, Y. Sato, S. Kashimoto, K. Chiba, J. Med. Chem.,2004, 47, 2097-2109.
- D.A. Burden, N. Oshereoff, Biochimica et Biophysica Acta.,1998, 1400, 139-154.
- R.M. Gengan, P. Pitchai, Elixir Appl. Chem., 2015, 86, 35088-35089.
- P. Pitchai, C. Uvarani, R.M. Gengan, P.S. Mohan, Indian J. Chem., 2013, 52B, 776.
- T.R. Makhanya, R.M. Gengan, P. Pitchai, A.A. Chuturgoon, C. Tiloke, A. Atar, J. Heterocyclic Chem.,2018, 55, 1193-1204.
- O. Meth-Cohn, Heterocycles., 1993, 35, 539-557.
- M.M. Ali, Tasneem, K.C. Rajanna, P.K.S. Prakash, Synlett., 2001, 2, 251-253.
- M.M. Ali, S. Sana, Tasneem, K.C. Rajanna, P.K. Saiprakash, Synth. Commun.,2002, 32, 1351-1356.
- R.A. Pawar, P.B. Bajare, S.B. Mundade, Heterocyclic compounds., 1991, 22, 43.
- A. Srivastava, R.M. Singh, Indian J. Chem. Sect. B, 2005, 44, 1868-1875.