Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
An Efficient and Alternative Large Scale Synthesis of Nardril (Phenelzine Sulfate)
Rajesh Kumar R1,2*, Prasada Raju VVNKV1 and Murthy C2
1Granules India Limited – R and D Center, Plot No.56, Road No.5, ALEAP Industrial Area, Pragathinagar, Hyderabad, India
2COEAMMPC and Division of Chemistry, Department of Science and humanities, Vignan's Foundation for Science, Technology and Research University (VFSTRU), Vadlamudi, Guntur, India
- *Corresponding Author:
- Rajesh Kumar R
Granules India Limited – R and D Center
Plot No.56, Road No.5, ALEAP Industrial Area, Pragathinagar, Hyderabad, India
Abstract
Facile and efficient,large scale synthesis of Nardril (Phenelzine sulfate) (5) can be prepared by alternative method from Phenethylamine (1) and 3, 3-pentamethylene oxaziridine (2) in the presence of acid catalyst. The main advantage of this method is low cost, easy work up and gave high yield of product. This method is very favorable for large scale synthesis of nardril in pharmaceutical industries.
Keywords
Nardril, Monoamine oxidase, Neurotransmitter, Noradrenaline, Psychiatric disorders.
Introduction
Monoamine oxidase (MAO, EC 1.4.3.4) is an enzyme that oxidizes many monoamines, including important neurotransmitter amines, such as noradrenaline (NA), dopamine, and 5-hydroxytryptamine (5-HT) [1]. Numerous MAO inhibitors have been synthesized and evaluated, and some of them have proven to be useful for treatment of psychiatric disorders such as depression, panic disorder, and social phobia [2,3]. Phenelzine, a non-selective MAO inhibitor that inhibits both the A and Bisozymes of MAO, has been used clinically for such purposes for many years [4].
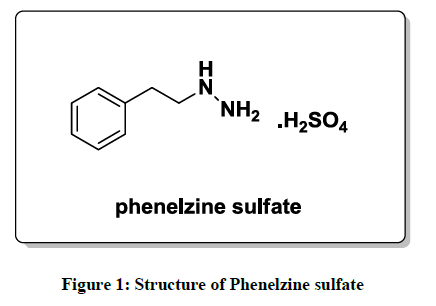
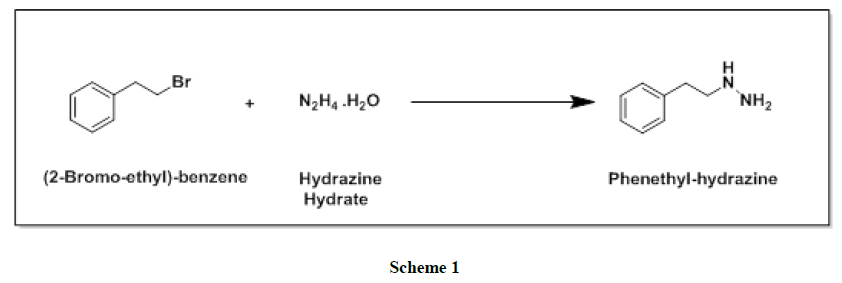
Phenelzine and its salts are an irreversible and nonselective and monoamine oxidase inhibitors (MAOI) of the hydrazine chemical class which is used as an antidepressant and anxiolytic. From the last few decades several methods have been reported in the literature for the preparation of phenelzine sulfate (Figure 1). One of the reported synthesis [5] of phenelzine proceeds in overall 77% yield starting from phenethyl bromide with 75% hydrazine hydrate as shown in Scheme 1.
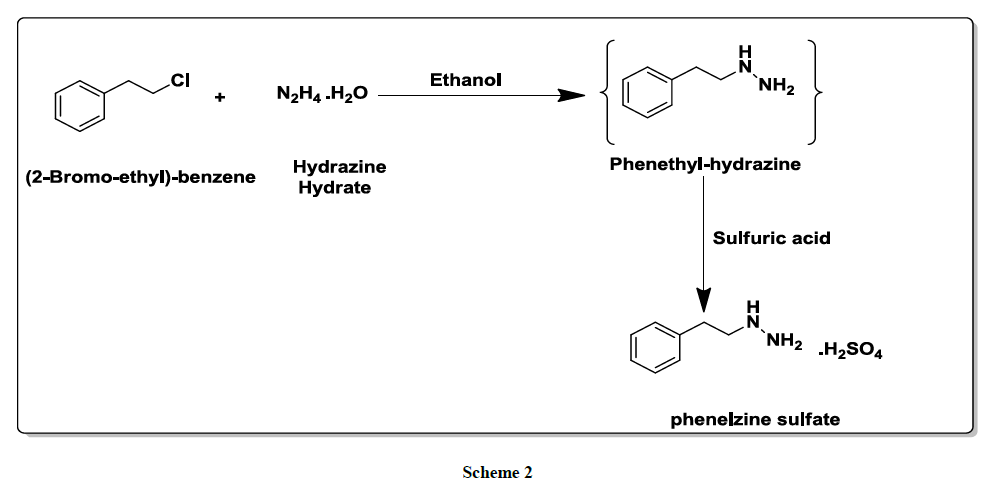
In a related procedure [6], an alternative preparation of phenelzineinvolves the reaction between phenethyl; chloride and hydrazine hydrate in ethanol followed by its salt formation using sulphuric acid (Scheme 2).
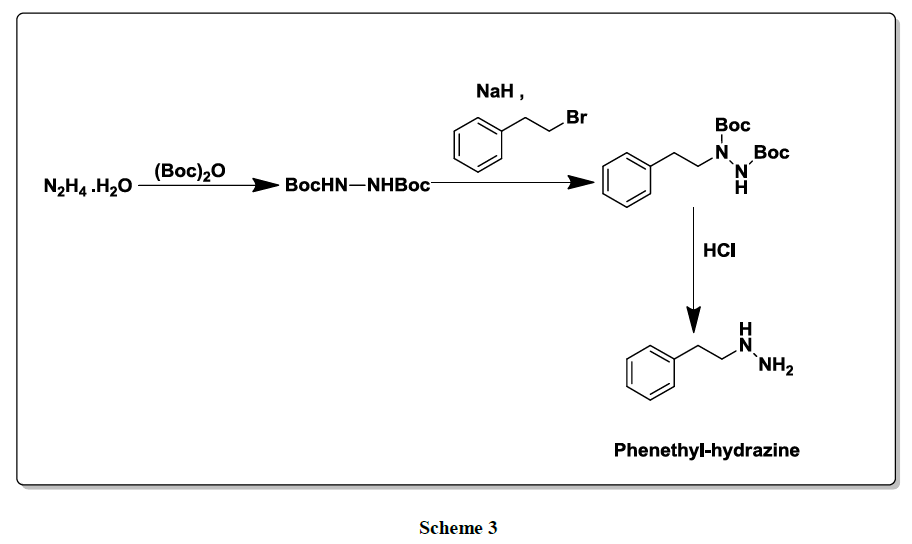
Another literature method [7] involved Boc protection of hydrazine to form the di Bochydrazide which on reaction with phenethyl bromide in presence of sodium hydride followed by deprotection of Boc with HCl affords Phenelzine in good yield (Scheme 3).
Material and Methods
Melting points are uncorrected and were determined on a Buchi Melting Point apparatus. 1H-NMR spectra were obtained in CDCl3 using TMS as internal standard on Brucker spectrometer at 300 MHz. Mass spectra were recorded on Shimadzu LCMS-QP8000, LCMS and AB-4000 Q-trap LC-MS/MS. The reaction was monitored by TLC on silica gel plates.
Preparation of N-cyclohexylidene-N’-phenethyl hydrazine (3)
To a suspension of Phenethyl amine (337.5 g, 1.5 mol) in toluene (3400 ml) 3,3-pentamethylene oxaziridine (oxaziridine content: 4 m/m %, 5000 ml, l. [delta] moles) are added in about 5 min, at a temperature of 90ºC. The reaction mixture was allowed to react at the same temperature for 30 min, Distilled solvent completely from reaction mixture. Product: 403.7 g (84%) of Oily residue.
Preparation of phenethyl hydrazine (4)
A solution of N-cyclohexylidene-N’-phenethyl hydrazine (3) (403.7 g, 1.26 mol) in 20 % hydrochloric acid (2500 ml) was stirred at an elevated temperature, washed with toluene to remove the impurities, then pH of the solution is adjusted to 8.0 with potassium carbonate solution and extracted with Isopropyl ether. From the obtained product with Isopropyl ether, Solvent was distilled of completely. Product: 267.7 g (87 %) of Oily residue.
Preparation of phenethyl hydrazine sulfate salt (phenelzine sulfate) 5
A solution of phenethyl hydrazine (4) (403.7 g, 1.26 mol) in isopropyl alcohol (2500 ml) is stirred at an 0-5ºC, added sulfuric acid in Isopropyl alcohol, stir for ½ h, Added n-Heptane to the solution and obtained solid product was filtered & washed with Isopropyl alcohol. Yellowish White solid, m.p. 164-166ºC; 1H-NMR (CDCl3): 7.25-7.34 (m, 5H), 3.32-3.37 (t, 2H), 2.93-2.98 (t, 2H); Mass (ES): m/z= 137.22.
Result and Discussion
However, most of the phenelzine synthetic procedures are suffer from several disadvantages from a commercial production stand point such as (a) cost effectiveness, (b) difficulty in handling the 2-halo ethyl benzenes on a large scale which requires precaution to avoid exposure to moisture, (c) isolation (drying operations) of intermediates and the work-up procedures which lead to increased cycle time and hence increase in the manufacturing cost. To overcome the above difficulties a novel method is required which can be useful for the preparation of phenelzine in large scale.
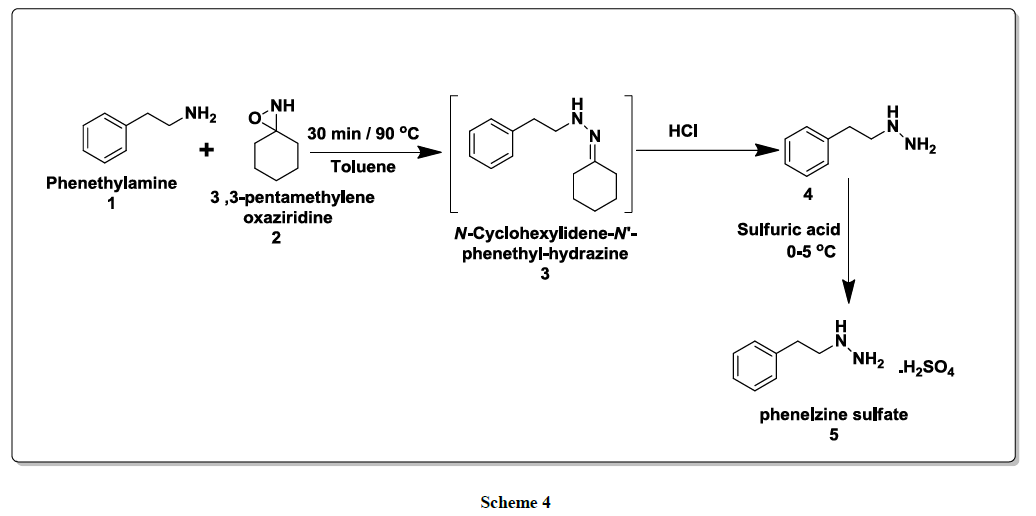
As illustrated in scheme 4, the reaction of Phenethylamine (1)with 3,3-pentamethylene oxaziridine (2)in toluene at 90ºC for 30 min led to the in situ formation of N-cyclohexylidene-N’-phenethyl hydrazine (3)intermediate under catalyst free condition. Then,to this reaction mixturewas added 20% hydrochloric acid and stirred for 1-2 h and neutralize the reaction mixture with potassium carbonate solution and extracted with Isopropyl ether. Processing the reaction mixture leads to the isolation of phenethyl hydrazine (4). Furthermore solution of phenethyl hydrazine was stirred with sulfuric acid at 0ºC-5ºC for 30 min gave final compound phenethyl hydrazine sulfate salt (phenelzine sulfate) (5). Structure of this product was assigned on the basis of its spectral properties -IR, NMR and Mass spectra (for details, please see the Experimental Section).
Based on the above succefull results, various green and organic solvents are screened for the formation 2 from 1. However in this all solvents toluene at 90ºC for 30 min was found to be the best method giving 2 in quality and yield (≥ 84%) (Table 1: Entry 1).
| Entry | Solvent | Temperature °C | Time (min) | 5a (%) |
|---|---|---|---|---|
| 1 | Toluene | 90 | 30 | 84 |
| 2 | Glycerol | 100 | 30 | 70 |
| 3 | PEG-600 | 100 | 30 | 72 |
| 4 | Ethylene glycol | 100 | 30 | 70 |
| 5 | Water | 100 | 30 | 61 |
| 6 | Methanol | 70 | 30 | 63 |
| 7 | Ethanol | 70 | 30 | 65 |
| 8 | DMF | 90 | 30 | 68 |
| 9 | THF | 90 | 30 | 68 |
| 10 | Acetonitrile | 70 | 30 | 66 |
| 11 | 1,4 dioxane | 80 | 30 | 71 |
Table 1: Effect of solvents for formation of 2 from 1
Conclusion
In summary, a practical and new synthetic have been developed for the synthesis of Nardril (Phenelzine sulfate) in toluene. The main advantage of this synthetic method is low cost, less reaction time, easy workup and isolation of product is simple with high yield.
Acknowledgement
The authors are indebted to the authorities of Cosmic Discoveries, Institute of Lifesciences, and University of Hyderabad for providing laboratory facilities.
References
- K.F. Tipton, P. Dostert, M.S. Benedetti, Monoamine oxidase and disease, Academic, London, 1984.
- C. Alec, The lancet., 1963, 281, 79.
- K.R.R. Krishnan, A.F. Schatzberg, C.B. Nemeroff, In The American psychiatric associationtextbook of psychopharmacology., 1998, 239.
- G.B. Baker, L.J. Urichuk, K.F. McKenna, S.H. Kennedy, Cell. Mol. Neurobiol.,1999, 19, 411.
- R.T. Coutts, A. Mozayani, F.M Pasutto, G.B. Baker, T.J. Danielson, J. Res. Commun. Chem. Pathol. Pharmacol.,1990, 67, 3.
- S.P. Sachin, V.P. Sachin, D.B. Vivek, Organic Preparations and Procedures International., 2011, 43, 260.
- L. Lei, J.U. Liana, S. Duff, et al. Bioorg. Med.Chem.Lett., 2001,11, 2715.