Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 8
Acute Toxicity Test of Standardized Ethanol Extract of Gandasuli Rhizome (Hedycium coronarium) and histopathology of organs in white mice male (Mus muculus)
Fajrian Aulia Putra1* and Netty Suharti22Faculty of Pharmacy, Andalas University, Anay Lestari Complex Block C No 7, Kuranji, Padang, Indonesia
Fajrian Aulia Putra, Faculty of Medicine, Fort de Kock University, Jl Dr Hamka no 101, Bukit Surungan, Padang Panjang, Indonesia, Email: fajrianauliaputra15@gmail.com
Received: 11-Aug-2022, Manuscript No. dpc-22-71703; Editor assigned: 13-Aug-2022, Pre QC No. dpc-22-71703; Reviewed: 20-Aug-2022, QC No. dpc-22-71703; Revised: 23-Aug-2022, Manuscript No. dpc-22-71703; Published: 30-Aug-2022, DOI: 10.4172/0975-413X.14.8.55-60
Abstract
Gandasuli (Hedycium coronarium.) is one of the native plants of Indonesia which It has been used as medicine by generations of people from generation to generation. This study aims to determine the acute toxicity of standardized ethanol extract of gandasuli rhizome (Hedycium coronarium) against male white mice, Swiss webster strain. The method used is to determine the value of Ld 50 using the Thompson-weil method. In this study, the parameters observed were the toxicity value of the standardized ethanol extract of gandasuli rhizome based on the Ld 50 value, histopathology of the liver and kidneys. A total of 25 male Swiss Webster white mice were randomly divided into 5 groups, namely group 1 as a control, group 2 treated with ethanol extract of gandasuli rhizome at a dose of 4 g/KgBW, group 3 at a dose of 8 g/Kg BW, group 4 with a dose of 16 g/Kg BW and group 5 with a dose of 32 g/Kg BW. The results of observations for 24 hours found that animals died in groups 1,2,3,4 and 5 respectively, namely 0,0,0,3 and 5 of 5 test animals, 14.92 mg/KgBB which are categorized into the practically non-toxic category because they are in a dose range of 5 g/KgBW – 15 g/KgBW. Observation of acute toxicity was continued by observing the histopathological state of the organ within 24 hours. The organs observed were the kidneys and liver which were given toxic doses. Based on the results of histopathological observations of the liver and kidneys of mice, it showed the occurrence of liver cell necrosis at a toxic dose of 32 mg/KgBW and in the kidneys showed bleeding.on the interstitial between tubule and edematous tubular cells.
Keywords
Gandasuli; Hedycium coronarium; Acute toxicity; LD50; Histopathology
INTRODUCTION
Herbal medicine is still used as the main treatment in developing countries and can be a solution for the use of chemical drugs because herbal medicines are more acceptable, more affordable, and have mild side effects.
Indonesia is famous as a country rich in medicinal plants, one of which is gandasuli (Hedycium coronarium J. Koening) which is a plant native to Indonesia from the Zingeberaceae family. The content of active compounds in gandasuli has been shown to have antioxidant and anti-inflammatory effects [1].
Although this plant has a myriad of benefits in terms of treatment, of course this plant also has harmful effects or deadly side effects. Philippus Aureolus Theophratus Bombast von Hohenheim states that everything that is efficacious as medicine is poison, only the dose that determines it is classified as non- toxic [2].
One of the requirements for a natural ingredient or medicinal plant to be developed is that it must be proven safe for consumption. To determine the toxic potential of a medicinal plant can be done with a toxicity test. The oral acute toxicity test is one of a series of toxicity tests that can be carried out to determine the risk of exposure of a substance to humans (BPOM, 2014).
Parameters observed in the acute toxicity test were changes in body weight, clinical symptoms, biochemical parameters, target organ histopathology, death and other general effects or specific effects.
Currently there are no reports on the level of safety in the use of gandasuli plants; therefore it is important to carry out acute toxicity testing of this plant.
Acute toxicity test is part of a preclinical test designed to measure the toxic effect of a compound. Acute toxicity refers to the toxic effects that occur after oral administration of a single dose within 24 hours. LD50 is the dosage that can cause the death of 50% of test animals and is obtained by statistical calculation of Lethal Dose or LD50 is a statistical measure after a single dose that is often used to express the level of a toxic dose as quantitative data. While clinical symptoms, physiological symptoms and toxic mechanisms as qualitative data [3].
In determining the value of LD50 One method that can be used is the Thompson- Weil method where this method uses a list of LD calculations50 in the Thomson-weil biometric table. This method was chosen because it has a fairly high level of confidence, accurate results, and does not require a large number of experimental animals.
RESEARCH METHODS
Tools and materials
The tools used in this research are, Rotary evaporator, Erlemenyer, Porcelain Exchange, Oven, Forness, sonde needle, mouse scales, mortar and stamper, filter paper, spatula, measuring cup, dropper pipette, analytical scale drop plate, surgical instrument and erlemeyer.
The material used is the rhizome of the gandasuli plant (Hedycium coronarium), Quarcetin, Gallic Acid, ethanol 96%, aquadest, glacial acetic acid, and Na CMC, chloroform (CHCl3), aquadest, FeCl3, HCl(p), H2SO4(p), anhydrous acetic acid, ammonia chloroform, H2SO4 2N, and Mayer's reagent.
Experimental Animals
The experimental animals used were 30 healthy male white mice aged 7-9 weeks with a body weight of 20-35 grams and then acclimatized for 7 days. Animals are declared healthy if during the acclimatization process the test animals do not show weight deviations of more than 10%.
In this study, the parameters observed were the death of the test.
Procedure
Sampling and Identification
The samples taken in this study were all parts of the fresh gandasuli rhizome plant obtained from the Sicincin area, District 2x11 Enam Lingkung, Padang Pariaman Regency, and West Sumatra Province. Identification of gandasuli rhizome samples (Hedycium coronarium) taxonomically conducted at the Andalas Herbarium (ANDA) Department of Biology, Faculty of Mathematics and Natural Sciences, Andalas University.
Manufacturing of Gandasuli Rhizome Ethanol Extract
Gandasuli rhizome was cleaned of impurities by washing with water, and then the sample was finely chopped and weighed as much as 1 kilogram. The sample was soaked (Maceration) in 96% ethanol for the first 6 hours while stirring occasionally, then allowed to stand for 18 hours. After that, separate the maserate by precipitation, repeat the filtration process at least 2 times with the same type and amount of solvent, then the maserate is evaporated withRotary evaporator until a thick extract is obtained.
Extract Suspension Manufacturing
The standardized ethanol extract of gandasuli rhizome was weighed according to the predetermined dose by preliminary testing, and the dose range to be used was obtained, namely the smallest dose of 4 g/KgBW, 8 g/KgBW, 16 g/KgBW and 32 g/KgBW., the extract was ground and then mixed with 1% w/v NaCMC solution which was developed with hot water and then mixed until homogeneous and added with distilled water.
Dosing
The dose used was based on a preliminary test that was carried out on the ethanol extract of gandasuli rhizome which was given orally to Swiss-Webster male white mice, and the results obtained that the dose did not cause death at the highest dose of 8000 mg/kgBW, the second dose was 4000 mg/kg. kgBW and 8000 mg/kg were found to have no toxic effects. In the preliminary test, first, the lowest dose was tested, namely 3000 mg/kg.BW.
Grouping of test animals
The 25 mice were randomly divided into five treatment groups, namely one control group which was given 1% NaCMC and four treatment groups which were given a dose of extract so that each group of test animals consisted of 5 male mice (Table 1).
Table 1: Grouping of test animals| No | Group | Quantity Animal | Treatment |
|---|---|---|---|
| 1 | Control | 5 | Given Na CMC 1% |
| 2 | 1 dose | 5 | The standardized extract was given suspended in 1% Na CMC at a dose of 4 g/kgBB |
| 3 | 2 dose | 5 | The standardized extract was given suspended in 1% Na CMC at a dose of 8 g/kgBB |
| 4 | Dose 3 | 5 | The standardized extract was given suspended in 1% Na CMC at a dose of 16 g/kgBB |
| 5 | Dose 4 | 5 | The standardized extract was given suspended in 1% Na CMC at a dose of 32 g/kgBB |
Acute toxicity test in test animals
25 experimental animals were fasted for approximately 18 hours but were still given water. Animals were weighed and grouped into 5 groups and each consisted of 5 tails. The administration of the ethanol extract of gandasuli rhizome was carried out in stages based on the prescribed dose.
In the acute toxicity test LD50, each treatment group was given a standardized ethanol extract of gandasuli rhizome which had been suspended with 1% NaCMC orally using a probe with different dose levels, namely 4 groups of dose levels and 1 control group. Mice were observed for 1-4 hours to see any visible toxic symptoms. Observations were made again 24 hours after dosing by counting the number of mice that died in the experimental group.
The mortality pattern of the test animals was entered into the Thomson-weil equation using the F value from the Thomson-weil biometric table.
Log m = log D + d(f + 1)
Information
m = LD value50
D = smallest dose used
d = log of dose multiple
f = a value in Weil's table, due to
a certain number of deaths
Histopathological examination of the organs of mice
Animals that die are seen for organ damage that occurs within 24 hours, the 2 most important organs are taken, namely the liver and kidneys, and the damage caused to these organs after being given a toxic dose, which is compared with the state of the organs of animals that are not given extract samples).
Kidney and liver histopathological preparations were made using the following procedure.
Fixation
The kidneys and liver are immersed in the solution formal neutral buffern (BNF) 10%. Fixation aims to avoid digestion of tissue by enzymes (autolysis) in the gaps or by bacteria and to maintain organ structure.
Dehydration
The dehydration process was carried out using alcohol with graded concentrations consisting of 70%, 80%, 90%, 95% and absolute alcohol. Further clear (clearing) by inserting the preparation into xylol.
Immersion (Embedding) and printing (blocking)
Preparations that have been dehydrated are planted in molds that have been filled with liquid paraffin to the brim and then cooled at room temperature cold plate. The hardened mold is removed from the mold and the resulting block can be stored in the refrigerator until it is ready to be cut with a microtome.
Cutting
The preparations in paraffin blocks were cut using a microtome with a thickness of 5 m to form like a ribbon and placed on the surface of warm water to prevent folds of the tape. The next preparation is placed on the object glass and dried at room temperature. The preparations were then stained with hematoxylin-eosin (HE) for histopathological observations with electric microscope.
RESULTS AND DISCUSSION
Identification of gandasuli rhizome plants obtained from the Sicincin area, District 2 x 11 Six Lingkung, Padang Pariaman Regency, and West Sumatra Province. has been carried out at the ANDA herbarium, Plant Taxonomy Laboratory, Department of Biology, Faculty of Mathematics and Natural Sciences (FMIPA), Andalas University, Padang. The identification results stated that the sample used in this study was Gandasuli rhizome (Hedycium coronarium J. Koening) (Figure 1).
From a wet sample of Gandasuli rhizome which was weighed as much as 1 kg, a thick extract was obtained as much as 54.447 grams and the yield value was 5.44%. Examination of the sample was organoleptic, the thick extract of gandasuli rhizome was black-brown in color, sour taste was slightly bitter, and had a characteristic sharp smell. In testing non-specific parameters, where to support standardized data from the extract of Gandasuli rhizome, the value of 4.72% ± 0.08% for simplicia water content was obtained, 2.48% ± 0.41% for drying shrinkage value. The easurement of the water content of simplicia aims to see what % of the water content contained in the simplicia, because this value aims to provide a minimum limit or range of the amount of water content in the material, because the greater the water content in a simplicia, the greater the potential for the development of microorganisms in the simplicia. . While the drying shrinkage aims to provide a maximum limit (range) of the amount of compounds lost in the drying process [4,5].
In the process of standardizing this gandasuli rhizome extract, to ensure the quality of the simplicia (extract) is not contaminated with heavy metal contamination, it is continued by determining the total ash content and acid insoluble ash content, and the results are 7.02% ± 0.33% respectively., and 0.59% ± 0.37% this value is in accordance with the standards set in the pharmacopoeia, namely for the total ash content value of ≤ 8% and the total ash content of acid insoluble 1%. Extracts that have gone through the standardization process are tested by giving the extract orally to test animals and the acute toxicity value is obtained by calculating the LD value.50. In this test, we observed the death of the test animals within 24 hours and observed the pattern of death of the test animals from 5 test animals of male white mice.
The following are the results of observations of test animals within 24 hours after being given treatment (Table 2).
Table 2: Observation results of acute toxicity test (Lethal dose 50) ethanol extract of gandasuli rhizome (Hedycium coronarium.)| No | Group | Dose (g/KgBB) | Number of Mice | Dead | Information |
|---|---|---|---|---|---|
| 1 | Control (-) | - | 5 | 0 | - |
| 2 | 1 dose | 4 g/KgBB | 5 | 0 | - |
| 3 | 2 dose | 8 g/KgBB | 5 | 0 | - |
| 4 | Dose 3 | 16 g/KgBB | 5 | 3 | Mortality >50% |
| 5 | Dose 4 | 32 g/KgBB | 5 | 5 | 100% death |
| Note: The above results can be found for the LD 50 value by entering it into the Thompson-Weil equation.With F value: 0.90000, F value is obtained from the biometric table value Thomson-weil | |||||
For the value of toxicity in this case the measurement of LD50 (lethal dose) using the Thomson-weil method where this method has a high level of confidence and the test method is quite easy, namely using a biometric table Thompson-Weil, From the observations, the dose value of LD50 with a value of 14.92 g/KgBW was obtained by conducting preliminary testing first to find the maximum dose that caused 0% death and the minimum dose that caused 100% death of the test animal [6,7].
The results of the LD50 value are categorized into the practically non-toxic category because they are in the dose range (5 g/KgBW – 15 g/KgBW).
To support the toxicity data obtained, histopathology of target organs such as kidneys and liver was carried out in test animals that died within 24 hours to see the acute toxic effects caused by the extract if given in toxic doses 16 g/KgBW. And the results were obtained.
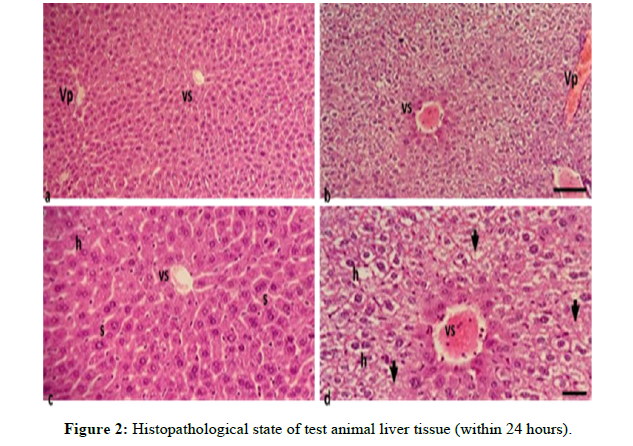
Animals that died at the highest or toxic doses were organ harvested and histopathological observations were carried out to see the state of organ damage in the test animals, this is supporting data to see whether the effects can be detected. Caused by the ethanol extract of gandasuli rhizome (Hedycium Coronarium J. Koening) on the kidneys and liver (Figure 2).
Information
a,) Liver cells of normal test animals with an objective magnification of 10 x 10μm . scale
b,) Toxic dose tested animal liver cells with an objective magnification of 10 x 10μm . scale
c,) Liver cells of normal test animals with an objective magnification of 40 xScale 10μm
d,) Toxic dose tested animal liver cells with an objective magnification of 40 xScale 10μm Vs = Central Vein Vp = Portal Vein h = Hepatocytes s = Sinusoid
Figure 3 (a, and c) show liver tissue with fine granular eosinophilic cytoplasmic hepatocytes, arranged in trabeculae with sinusoids in between, and also visible central veins in the central lobes. Administration of high doses of gandasuli extract showed liver tissue damage Figure 3 (b and d) characterized by swollen liver cells, hydropic degeneration with clear vacuolated cytoplasm, dilated central veins and portal blood vessels filled with erythrocytes, and narrow sinuses. Some cells appear necrotic with missing nuclei (arrows) [8].
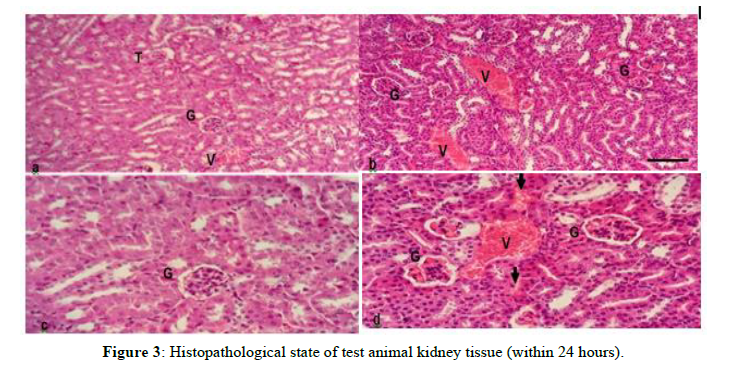
This indicates that the extract with a toxic dose causes damage to the liver based on the histopathological condition of the liver of mice. In addition to the liver organ, there is also damage observed in histopathological conditions on kidney organs in test animals, such as kidney organs in test animals (Figure 3).
Information
a,) Kidney cells of normal test animals with an objective magnification of 10 x Scale 10μm
b,) Toxic dose test animal kidney cells with an objective magnification of 10 x 10μm scale
c,) Kidney cells of normal test animals with an objective magnification of 40 x Scale 10m
d,) Toxic dose test animal kidney cells with objective magnification of 40 x Scale 10m G = Glomerulus with Tubuli V = Blood Vessel T: Tubuli
Figures (a, and c) represent the histology of normal experimental animal kidney tissue; while (b, and d) represent the histology of kidney tissue with toxic doses of gandasuli ethanol extract Hedychium coronarium J. Koening. Kidney tissue with cortex consists of tubules (T) and glomerulus (G). The effects after administration of extracts with toxic doses that caused death in test animals showed damage to kidney tissue which was characterized by tubular cells that were partially edematous in tubules with a slightly narrower lumen, widened blood vessels, accompanied by bleeding/hemorrhagic areas (arrows) in the interstitial space between tubules. Glomeruli with dilated capillaries containing erythrocytes, and inflammation occurs in the glomerulus.
The reason for the researchers to test this organ is because the kidney is the organ where metabolism and drug elimination processes occur.
CONCLUSION
Based on the results of the study, it can be stated that the administration of ethanol extract of gandasuli rhizome (Hedycium coronarium. J Koening) which has been standardized proven to be safe with Ld. values50 (lethal dose) 14.92 g/KgBB where this value is included in the Practically non-toxic category. And the discovery of organ damage to the kidneys and liver after being given toxic doses to test animals as seen from the histopathological observations of the organs.
References
- Food and Drug Supervisory Agency of the Republic of Indonesia. 2014.
- Ministry of Health of the Republic of Indonesia. 1989.
- Indonesian Ministry of Health. 1995, p. 2-5.
- Jenova R. Diponegoro University. 2009.
- Mescher AL. Junqueira's Basic Histology: text & atlas. 2011.
- Pooja P, Dixit AK. Int J Chinese Medicine. 2017, 1(2): p. 49-61.
- Shargel L and Yu A. Applied Biopharmaceutics and Pharmacokinetics. 1999.
- Wirasuta. I Made Agus Gelgel, Rasmaya Niruri. General Toxicology. 2007.
- Wirasuta MAG and Niruri R. General Toxicology. Bali. 2016.