Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
A Simple and Efficient, Silica Supported Oxon Catalyzed One-pot Synthesis of 2-Aryl-Benzimidazoles
Kumaraswamy G1*, Brahmeshwari G2, Ravichander M1, Jagadeesh Babu K2 and Srinivas B3
1Department of Chemistry, Mahatma Gandhi Institute of Technology, Hyderabad-500075, India
2Department of Chemistry, Kakatiya University, Warangal-506009, India
3Department of Chemistry, Chaitanya Postgraduate College (Autonomous), Warangal-506001, India
- *Corresponding Author:
- Kumaraswamy G
Department of Chemistry
Mahatma Gandhi Institute of Technology
Hyderabad-500075, India
Abstract
A simple and efficient method for the synthesis of 2-arylbenzimidazole has been described involving the reaction of orthophenylene diamine with various primary alcohols using silicon supported Oxon as a catalyst. This method also describes mild reaction conditions, short reaction times, simple procedure, good yields and eco-friendly features.
Keywords
One pot synthesis, 2-Aryl benzimidazoles, Silica supported oxone
Introduction
In recent years, chemistry of benzimidazole moiety has been of great interest in modern drug discovery. The organic compounds, specifically with nitrogen containing heterocyclic ring system exhibit a wide range of pharmaceutical applications associated with a wide variety of medicinal, biological activities [1,2]. Substituted benzimidazole derivatives have wide spread applications in diverse therapeutic areas including antimicrobial [3], analgesic [4], antibacterial [5], antitumor [6] antifungal [7] and anti-inflammatory drugs [8].
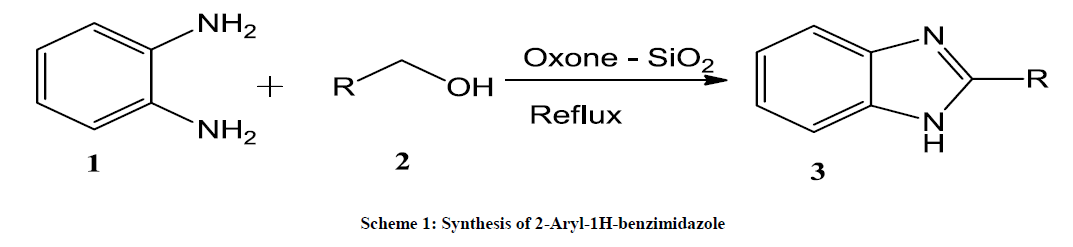
There are many methods for synthesis of benzimidazole, one of the classical methods is coupling of orthophenylene diamines with carboxylic acids or their derivatives [9], which requires strongly acidic conditions and high temperature and the other method is the oxidation of benzimidazoline intermediates that are generated from the condensation of orthophenylene diamine and aldehydes [10]. Although, there is a demand for efficient methods for the synthesis of Benzimidazole, herein, it is reported a new strategy for the synthesis of benzimidazoles by coupling orthophenylene diamine and primary alcohols as the reactants at room temperature using oxon-SiO2 as a catalyst under simple conditions.
Materials and Methods
Melting points of the synthesized compounds were determined in open capillary tubes and were uncorrected. Reaction progress was observed by Thin Layer Chromatography (TLC) plates. 1H-NMR (300 Mz) and 13C-NMR (100 Mz) Bruker NMR instrument in Dimethyl Sulfoxide (DMSO) using Tetramethylsilane (TMS) as internal standard. Chemical shifts (δ) are expressed in ppm. Perkin Elmer BX series FT-IR was used to record IR spectra, Elemental analysis was performed on a PerkinElmer 240 CHN analyzer.
Experimental section
Procedure for synthesis of silica supported oxon catalyst
Oxon® (4.56 g, 0.003 mmol) was dissolved in 15 ml of hot water taken in 50 ml round-bottomed flask. To the hot solution was added silica gel (230-400 meshes, 2.9 g) with vigorous stirring. The resultant oxon supported with silica gel was dried in oven at 80°C for 10 h to obtain a white freeflow powder.
General procedure for the synthesis of 2-Aryl-1H-benzimidazole
Ortho phenylene diamine (0.226 g, 1.66 mmol) was dissolved in CH3CN (5 ml) and was added, with oxone (0.663 g, 1.08 mmol, 0.65 equiv). A solution of benzyl alcohol (0.213 g, 1.75 mmol, 1.05 equiv) in CH3CN (1 ml) was added drop wise and stirred the reaction mixture for about 30 min. After completion of the addition of solution, stirring was continued for an additional 2 h. The progress of the reaction was followed by TLC, the mixture was treated with ethyl acetate and then concentrated in vacuo. The residue was purified by silica-gel column chromatography with ethyl acetate/hexane (1:4) to afford pure 2-aryl benzimidazole (Scheme 1 and Table 1). The resulting precipitate was collected by filtration, washed with H2O and dried.
| Entry | Alcohol | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | C6H5-CH2OH | 3a | 4.5 | 85 |
| 2 | 4-CH3-C6H4-CH2OH | 3b | 4.8 | 82 |
| 3 | 4-OCH3C6H4-CH2OH | 3c | 4.9 | 80 |
| 4 | 4-Cl-C6H4-CH2OH | 3d | 5.1 | 81 |
| 5 | 4-OH-C6H4-CH2OH | 3e | 5.7 | 78 |
| 6 | N(CH3)2-C6H4-CH2OH | 3f | 4.9 | 76 |
| 7 | C6H5-CH=CHOH | 3g | 4.3 | 77 |
| 8 | C6H11-CH2OH | 3h | 4.5 | 75 |
| 9 | C4H3-CH2OH | 3i | 4.6 | 74 |
| 10 | 4-NO2-C6H4-CH2OH | 3j | 5.2 | 72 |
Table 1: Synthesis of 2-aryl substituted benzimidazole
2-Phenyl-1H-benzimidazole (3a): (C13H10N2)-Paleyellow solid, M.P. 290-292oC. IR (KBr) λmax in (cm-1); 3371 (NH), 1586 (C=N), 1442 (C=C); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm) 12.63 (1H, s, NH), 8.18 (2H, m, Ar), 7.45-7.67 (m, 5H, Ar), 7.25 (2H, m, Ar); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm), 151.0, 143.5, 134.8, 130.1, 129.8, 128.7, 126.4, 122.4, 121.6, 118.8, 113.3. Found: C, 80.37; H, 5.16; N, 14.36. Cal for C13H10N2: C, 80.42; H, 5.19; N, 14.42%).
2-(4-Methylphenyl)-benzimidazole (3b): (C14H12N2)-White solid, M.P. 260-262°C. IR (KBr) λmax in (cm-1); 3353 (NH), 1565 (C=N), 3086 (CH); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm) 2.38 (3H, s, Me), 7.18 (2H, m, Ar), 12.80 (1H, s, NH), 8.16 (2H, m, Ar), 7.40 (2H, m, Ar), 7.65 (2H, m, Ar); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 143.8, 139.6, 129.5, 127.4, 126.4, 122.3, 121.6, 118.7, 111.1, 21.0; Anal. Calcd. For C14H12N2: C, 80.75; H, 5.80; N, 13.47. Found: C, 80.60; H, 5.85; N, 13.59.
2-(4-Methoxyphenyl)1-H-benzo[d]imidazole (3C): (C14H12N2O)-colorless solid, M.p. 223-225°C. IR (KBr) λmax in (cm-1); 1524 (C=N), 3347 (NH), 1085 (OCH3); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm) 12.11(1H, s, NH), 8.21 (2H, m, Ar), 7.68 (2H, m, Ar), 7.45 (2H, m, Ar), 7.20 (2H, m, Ar), 3.55 (3H, s, OMe); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 58.43, 115.7, 121.6, 126.3, 127.8, 139.6, 143.8, 154.80. Anal. Calcd. For C14H12N2O: C, 74.98; H, 5.39; N, 12.49. Found: 74.12; H, 5.10; N, 12.1.
2-(4-Chlorophenyl)-1H- benzo[d]imidazole (3d): (C13H9ClN)-White solid, M.p. 288-290°C. (KBr) λmax in (cm-1); 1624 (C=N), 3447 (NH), 780 (C-Cl); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm) 12.24 (1H, s, NH), 8.26 (2H, m, Ar), 7.58 (2H, m, Ar), 7.40 (2H, m, Ar), 7.25 (2H, m, Ar); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 131.92, 128.95, 127.54, 125.14, 123.16, 121.51, 115.7. Anal. Calcd. For C13H9ClN: C, 68.28; H, 3.97; N, 12.25. Found: 67.98; H, 3.30; N, 12.10.
2-(4-Hydroxyphenyl)-1Hbenzo[d]imidazole (3e): (C13H10N2O)-white solid, M.P. 254-256°C. IR (KBr) λmax in (cm-1); 3335 (NH), 1572 (C=N), 3570 (OH); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm 12.63 (1H, s, NH), 9.94 (1H, s, OH), 7.69-8.32 (4H, m, Ar) 6.89-7.52 (4H, m, Ar); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 158.9, 145.0, 138.8, 128.0, 121.4, 120.5, 115.4, 114.2. Anal. Calcd. For C13H10N2O: C, 74.27; H, 4.79; N, 13.33. Found: C, 74.18; H, 4.68; N.13.28.
4-(Benzo[d]imidazol-2-yl)-N,N-dimethylaniline (3f): (C15H15N3)-White solid, M.p. 273-275°C. IR (KBr) λmax in (cm-1): 3365 (NH), 1628 (C=N), 2922 (C-H); 1H-NMR (DMSO-d6, 300 MHz, δppm) 12.47 (s, NH), 8.01 (d, 2H,), 7.450-7.56 (m, 2H), 6.80-6.86 (d, 2H), 3.00 (s, 6H); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 152.12, 150.22, 143.01, 134.70, 126.52, 121.18, 121.16, 117.31, 110.60, 41.05. Anal. Calcd for C15H15N3: C, 75.93; H, 6.38; N, 17.71. Found: C, 74.98; H, 5.89; N, 17.48.
2-Styryl-1H-benzo[d]imidazole (3g): (C15H12N2)-white solid, M.p. 201-203°C. IR (KBr) λmax in (cm-1): 3377 (NH), 1674 (C=N), 1634 (C=C); 1H-NMR (DMSO-d6, 300 MHz, δ ppm) 12.12 (s, 1H), 6.40 (dd, 1H), 6.51 (d, 1H), 7.13- 7.52 (m, 7H), 7.71 (d, 2H); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 140.92, 134.95, 127.54, 125.14, 123.16, 121.51, 115.7 Anal. Calcd for C15H12N2: C, 81.79; H, 5.49; N, 12.72. Found C, 81.18; H, 5.08; N, 12.24.
2-Cyclohexyl-1H-benzo[d]imidazole (3h): (C13H16N2)-colorless crystal, M.p. 232-234°C. IR (KBr) λmax in (cm-1): 3355 (NH), 1622 (C=N); 1HNMR (DMSO-d6, 300 MHz, δ, ppm) 12.10 (s, 1H), 7.44-7.48 (m, 2H), 7.08-7.15 (m, 2H), 2.82 (t, 1H), 2.01-2.06 (m, 2H), 1.52- 1.83 (m, 5H), 1.21-1.442 (m, 3H); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 159.2, 143.2, 133.5, 121.2, 121.1, 112.2, 39.3, 31.1; Anal. Calcd. for C15H15N3: C, 76.93; H, 8.05; N, 13.98. Found: C, 76.68; H, 8.09; N, 13.48.
2-(Furan-2-yl)-1H-benzo[d]imidazole (3i): (C11H8N2O)-white solid, M.P. 286-288°C. IR (KBr) λmax in (cm-1): 3371 (NH), 1627 (C=N): 1HNMR (DMSO-d6, 300 MHz, δ, ppm) 12.24 (s, 1H), 8.05 (d, 1H), 7.46 (d, 2H), 7.12-7.18 (m, 3H), 6.75 (m, 1H); 13C-NMR (DMSO-d6, 100 MHz, δ, ppm) 143.6, 142.5, 132.3, 122.3, 120.6, 117.7, 112.3, 110.4. Anal. Calcd for C11H8N2O: C, 71.73; H, 4.38; N, 15.21. Found: C, 71.68; H, 4.39; N, 15.48.
2-(4-Nitro phenyl)1H-benzimidazole (3j): (C13H9N3O2)-Yellow solid, M.P. 316-318°C. IR (KBr) λmax in (cm-1) 3368 (NH), 1569 (C=N), 1524 (NO2); 1H-NMR (DMSO-d6, 300 MHz, δ, ppm) 13.20 (1H, s, NH) 8.32-8.37 (m, 2H, ArH), 7.72-7.98 (m, 2H, ArH), 7.24-7.56 (m, 2H, ArH), 6.95-7.12 (m, 2H, ArH); 13C-NMR (DMSO, 100 MHz, δ, ppm) 149.1., 147.9, 136.1, 129.6, 127.5, 124.4, 123.1, 120.0,. Anal. Calcd. For C13H9N3O2: C, 65.27; H, 3.79; N, 17.56. Found: C, 65.40; H, 3.83; N, 17.42.
Results and Discussion
In the present study, it has been discussed that the synthesis of various 2-aryl benzimidazoles by the condensation of orthophenylenediamine and primary alcohols using catalytic amount of Silica supported oxone as an eco-friendly catalyst. This method has great advantages including stability, eco-friendly, easy of transport, simple handling, and nontoxic nature [11-13]. In the reaction, a Primary alcohol was initially subjected to oxidation with oxon in various solvents by refluxing and the resultant aldehyde was subsequently transformed into the corresponding benzimidazole by coupling with orthophenylenediamine. Here the efforts are more focused on the synthesis of 2-aryl benzimidazoles by using Silica supported oxon as a catalyst and initially, the yield was not found to be more than 70% without using silica, even after the time is prolonged. Further, the reactions were carried out with supporting of silica, it has been noted that the efficacy of catalyst significantly increased and gave a high yield of products. The above reaction was carried out in various solvents such as Ethanol, acetonitrile, methanol, Tetrahydrofuran (THF), Dimethylformamide (DMF) (Table 2). It was found that acetonitrile is the best suitable solvent for the reaction and giving higher yield as compared to other solvents.
| Entry | Solvent (3a) | Oxone® | SiO2 | Time (h) | Yield(%) |
|---|---|---|---|---|---|
| 1 | Ethanol | 4.56g | - | 6.5 | 62 |
| 2 | Ethanol | 4.56g | 2.9g | 4.5 | 80 |
| 3 | Acetonitrile | 4.56g | - | 5.8 | 68 |
| 4 | Acetonitrile | 4.56g | 2.9g | 4 | 85 |
| 5 | DMF | 4.56g | - | 6.3 | 66 |
| 6 | DMF | 4.56g | 2.9g | 4.8 | 78 |
| 7 | THF | 4.56g | - | 6.2 | 60 |
| 8 | THF | 4.56g | 2.9g | 4.8 | 72 |
| 9 | H2O | 4.56g | - | 8.2 | 50 |
| 10 | H2O | 4.56g | 2.9g | 6.4 | 60 |
Table 2: Optimization of reaction conditions
The chemical structure of title compoundswere established on the basis of spectroscopic analysis. The synthesized compounds showed infrared absorption bands in the range from 3335 to 3371 cm-1 for (NH) functions, 1565-1586 cm-1 for (C=N). The 1H-NMR spectra of title compounds revealed the presence of signals at 12.63 ppm for NH proton, and the aromatic protons as a multiplet (H) in the range 6.80-8.36 ppm. 13C-NMR spectrum of compounds shows the signals in the range of 120.1 to 149.1 ppm which represents benzimidazole and phenyl ring.
Conclusion
The research study reported efficient method for synthesis of 2-aryl benzimidazoles derivatives from phenylene diamine and aromatic alcohols using catalytic amount of oxone as an oxidant. It is believe that, this method is a valid contribution to the existing methodologies and a new addition of the catalytic activity in the several organic syntheses.
Acknowledgement
The authors gratefully acknowledge to the Department of chemistry, Kakatiya University, Warangal for constant support during this research work.
References
- J.S. Kim, B. Gatto, C. Yu, A. Liu, L.F. Liu, E. La Voie, J. Med. Chem., 1996, 39, 992-998.
- T. Roth, M.L. Morningstar, P.L. Boyer, S.H. Hughes, J.R.W. Buckheit, C.J. Michejda, J. Med. Chem., 1997, 40, 4199-4207.
- N.C.D. Amit, Niraj, Med. Chem. Res., 2012, 21, 2579-2586.
- S. Dixit, P. Kumar sharma, N. Kaushik, Med. Chem. Res., 2013, 22, 900-904.
- K. Gullapelli, M. Thupurani, G. Brahmeshwari, Int. J. Pharm. Biol. Sci., 2014, 5, 682-690.
- S.A. Galal, A.S. Abdelsamie, M.L. Rodriguez, S.M. Kerwin, H.I. Diwania, Eur. J. Chem., 2010, 1, 67-72.
- K. Hasan, D. Riza, S. Nihat, S. Gunal, Asian J. Chem., 2009, 21, 6181-6189.
- L.P. Shi, Y.D. Ren, W. Lu, J.P. Zuo, J. Med. Chem., 1989, 49, 4790.
- P.N. Preston, Chem. Rev., 1974, 74, 279-314.
- K. Bahrami, M.M. Khodaei, I. Kavianinia, Synthesis., 2007, 547-550.
- J.P.Y. Kao, S. Muralidharan, P.Y. Zavalij, S. Fletcher, F. Xue, G.M. Rosen, Tetrahedron Lett., 2014, 55, 3111-3113.
- P.L. Beaulieu, B. Haché, E. von Moos, Synthesis., 2003, 1683-1692.
- B.R. Travis, M. Sivakumar, G.O. Hollist, B. Borhan, Org. Lett.,2003,5, 1031.