Review Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
A Review on Methods of Preparation and Biological Activities of Benzimidazolyl Chalcones
Rita Saini1* and Alka N Choudhary2
1Department of Pharmaceutical Sciences, Himalayan Institute of Pharmacy and Research, Dehradun, Uttarakhand, India
2Department of Pharmaceutical Sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India
- *Corresponding Author:
- Rita Saini
Department of Pharmaceutical Sciences
Himalayan Institute of Pharmacy and Research
Dehradun, Uttarakhand, India
Abstract
The diverse pharmacological activities of Benzimidazolyl chalcone derivatives such as antibacterial, antifungal, anticancer, anti-inflammatory, anti-tubercular, analgesic, insect anti feedant, Central nervous system activities, attract many researchers to develop efficient synthetic methods. The present reviews attempted to gather the various developments in synthesis and biological activities of Benzimidazolyl chalcone derivatives.
Keywords
Benzimidazolyl chalcones, Antibacterial activity, Anticancer activity, Antiinflammatory activity, Antitubercular activity, Insect anti feedant activity.
Introduction
Benzimidazole is regarded as a promising class of bioactive heterocyclic compounds that exhibit a range of biological activities [1]. Specifically, this nucleus is a constituent of vitamin-B12. This ring system is present in numerous antioxidant [2], antimicrobial [3], antihelmintics [4], anti- HIV, anticonvulsant [5], anti-inflammatory [6], antineoplastic [7], and antifungal activities [8]. The varied bioactivities exhibited by benzimidazoles, efforts have been made from time to time to generate libraries of these compounds and screened them for potential biological activities.
Benzimidazole derivatives on combining with chalcones have resulted in formation of compound possessing a large number of different biological activities [9,10]. The chemical profile of benzimidazolyl-chalcones was conceptualized according to pharmacochemical methodologies of juxtaposition of potential biological entities. The choice of these two entities (benzimidazole and phenylpropenone) is justified by their high intrinsic ability to induce biological activities of therapeutic interest. Due to versatility of these compounds chemists are mainly focused on their synthetic methods. Different derivatives of chalcone clubbed with benzimidazole nucleus as a prime motif were synthesized by different authors [11].
Different methods are reported in the literature for the preparation of Benzimidazolyl chalcones. Some classical methods are Claisen-Schmidt condensation, Suzuki reaction and Heck reaction [12]. Besides classical methods for synthesizing these chalcones, the green chemistry approach proposes as well a variety of examples. There are current different methods; these differ in conditions implying catalysts, solvents and times of irradiation [13,14].
Due to versatility of these compounds, we became interested in the study of different methods of synthesis of substituted Benzimidazolyl chalcone derivatives. This review summarizes the different synthetic methodology for different derivatives of substituted Benzimidazolyl chalcones along with their activity.
Synthetic schemes for Benzimidazolyl chalcones
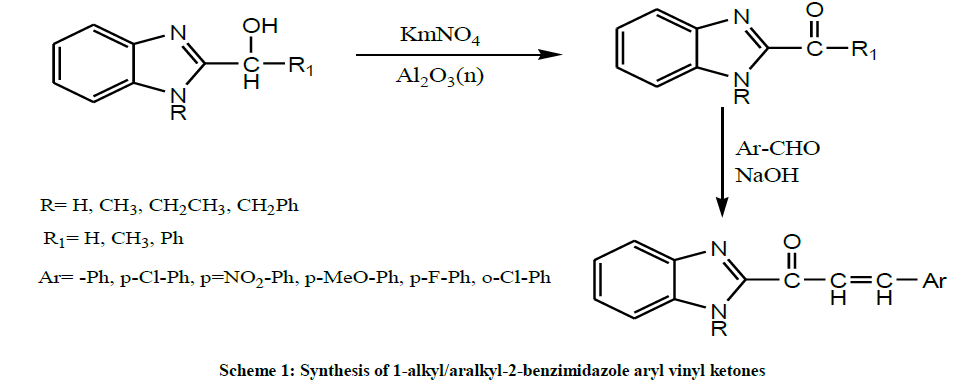
Dubey et al. in 2003 performed the oxidation of 1-alkyl/aralkyl-2(α-hydroxyalkyl/aryl) benzimidazoles with KMnO4 on neutral alumina under solid phase conditions give corresponding ketones, 1-alkyl/aralkyl-2-acyl-benzimidazoles. Further condensation with aromatic aldehydes in the presence of solid NaOH under solvent free conditions yields corresponding chalcones, 1-alkyl/aralkyl-2-benzimidazole aryl vinyl ketones (Scheme 1) [15].
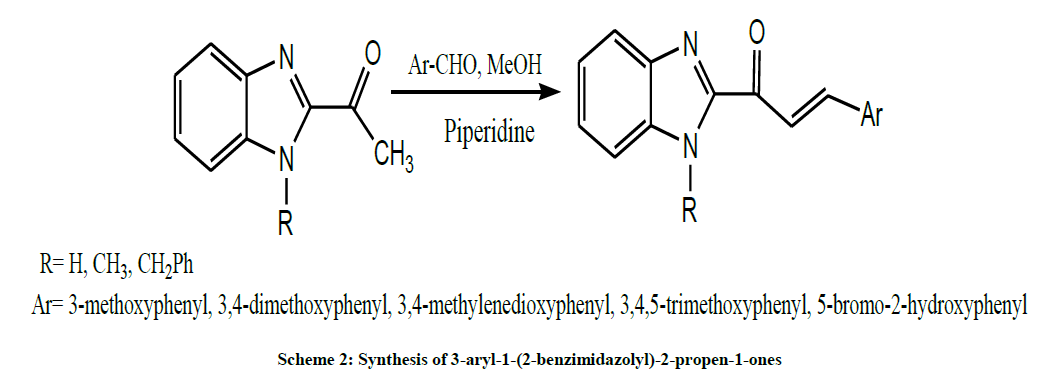
In 2004, Dumitrescu et al. synthesized 3-aryl-1-(2-benzimidazolyl)-2-propen-1-ones by the condensation of 2- acetylbenzimidazole with variously substituted aromatic aldehydes in the presence of catalytic amounts of piperidine (Scheme 2) [16].
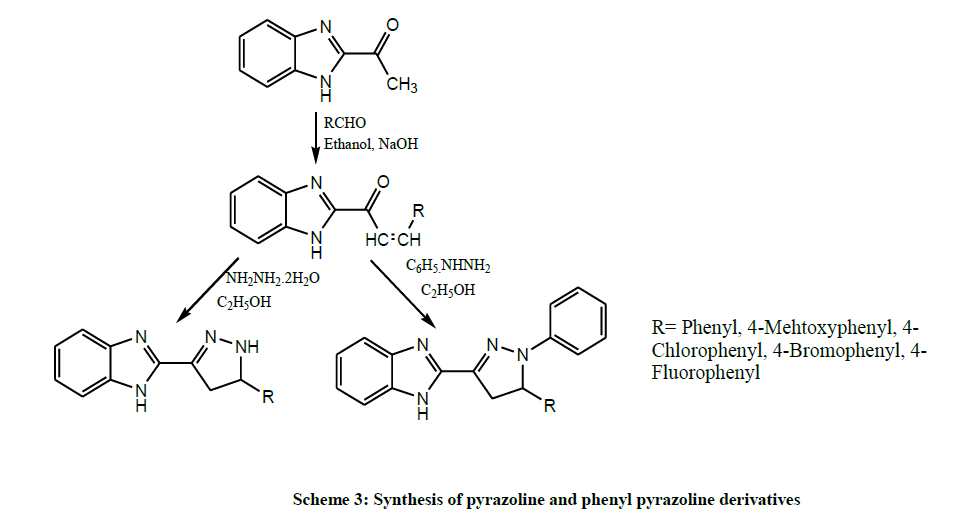
Shahar et al. allowed to react a series of 1-(1H-benzimidazol-2-yl)-3-(substituted phenyl)-2-propen-1-one with hydrazine hydrate and phenyl hydrazine in submitted reactions to get pyrazoline and phenyl pyrazoline derivatives (Scheme 3). All the compounds entered for screening of in vitro antibacterial activity. Compound 2-[5-(4- fluorophenyl)-1-phenyl-4,5-dihydro-1H-3-pyrazolyl]-1H benzimidazole was considered the best candidate of the series that could be a good starting point to develop new lead compounds in the fight against tuberculosis [17].
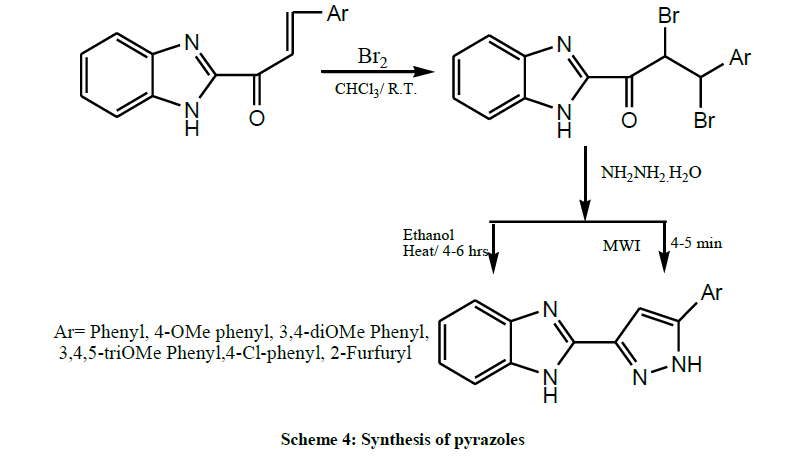
In the same year, the Benzimidazolyl chalcones (Scheme 4) were brominated by using bromine in chloroform at room temperature to get corresponding benzimidazolyl chalcone dibromides, further these dibromides were treated with hydrazine hydrate under refluxing condition, to get desired pyrazoles by Rajora et al. [18].
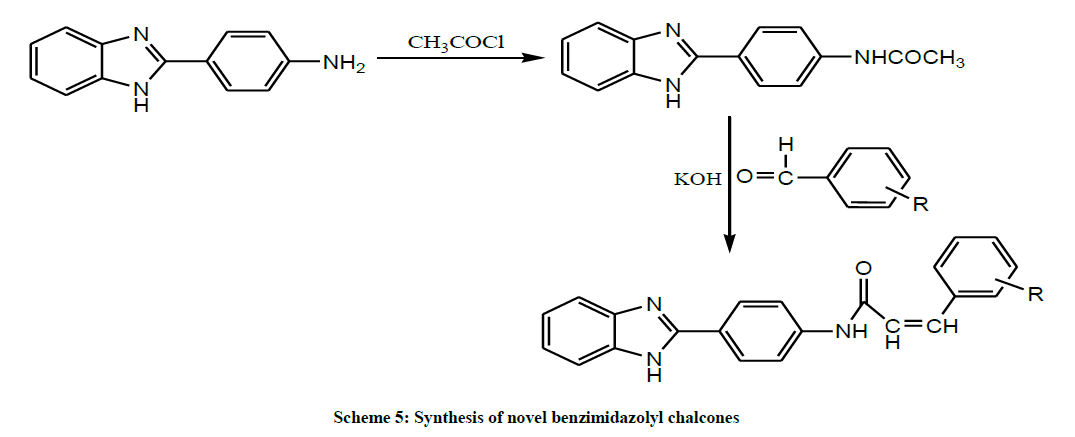
Later, Baviskar et al. prepared some novel benzimidazolyl chalcones by condensation of N-(4-(1H-benzo[d]imidazol-2-yl) phenyl) acetamide with aromatic aldehydes in presence of aqueous potassium hydroxide solution at room temperature (Scheme 5). All the compounds have been screened for antimicrobial activity by the cup-plate method. From the antimicrobial screening it was observed that all the compounds exhibited activity against all the organisms employed. Looking at the structure activity relationship, marked inhibition in bacteria was observed in the compounds bearing Ar = C6H5, 4-OCH3 C6H4, 4-OHC6H4, 3-FC6H4, 3-BrC6H4 substituent’s where as other compounds showed moderate to good activity. Fungicidal screening data also revealed that compounds bearing Ar = 2-NO2C6H4, 4-N(CH3)2C6H4, 4-OCH3C6H4 imparted maximum activity to the compounds, where as other compounds showed moderate to good activity [19].
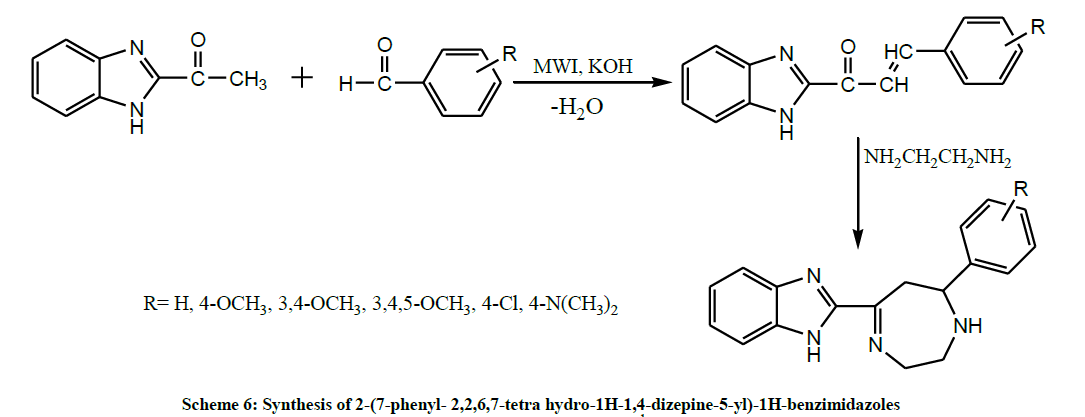
In 2010, Yadav et al. performed the reaction of substituted benzimidazolyl chalcones with ethylene-diamine afforded 2-(7-phenyl- 2,2,6,7-tetra hydro-1H-1,4-dizepine-5-yl)-1H-benzimidazoles (Scheme 6) by both MWI and conventional methods. The newly synthesized compounds were screened for the antibacterial and antifungal activity in vitro and results showed that all the compounds possess promising activity [20].
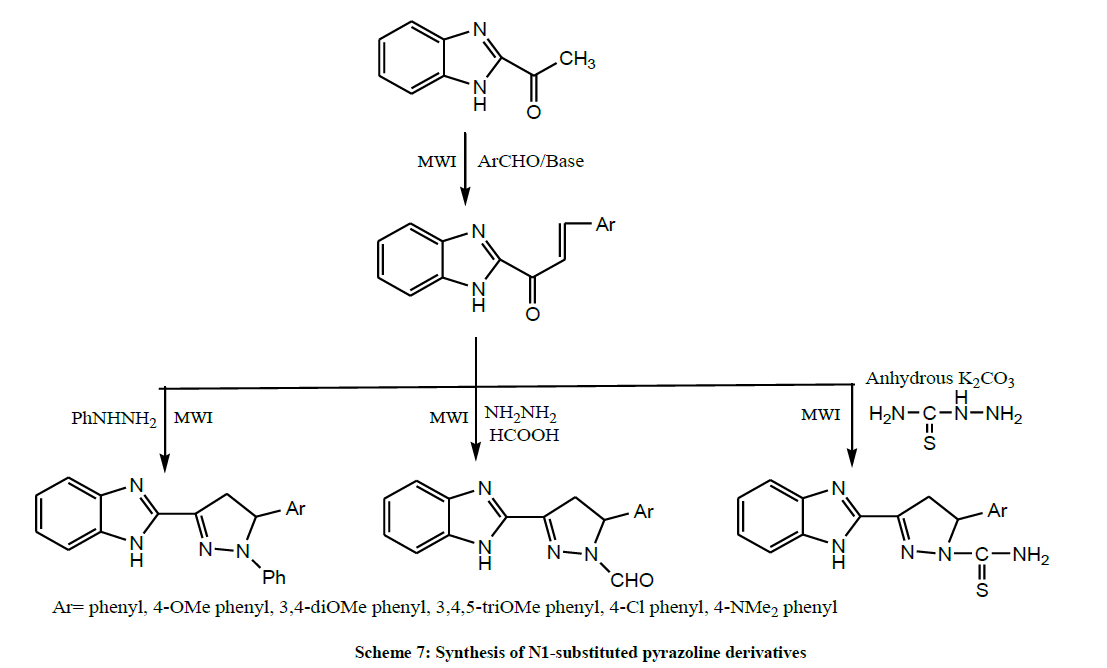
Srivastava et al. transformed the 1-Benzimidazolyl-3-aryl-prop-2-ene-1-ones into N1-substituted pyrazoline derivatives by the interaction with phenyl hydrazine, thiosemicarbazide and hydrazine hydrate in the presence of formic acid under solvent free microwave induced protocol (Scheme 7). Newly synthesized compounds have been evaluated for their antimicrobial activity. Some of the compounds have shown significant activity against the pathogens [21].
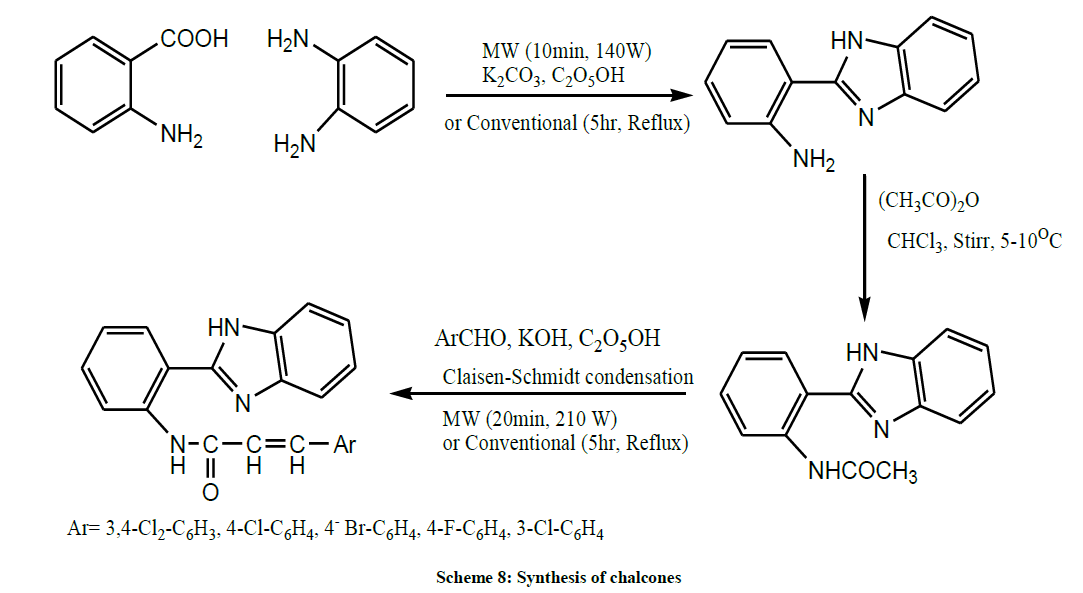
Sahoo et al. in the same year, treated anthranillic acid with orthophenylene diamine to produced benzimidazole. Further the acetylated product of benzimidazole undergoes condensation with aryl aldehyde to produce corresponding chalcones (Scheme 8). Both conventional and microwave irradiated synthesis of chalcone has been carried out to compare their yield and reaction time [22].
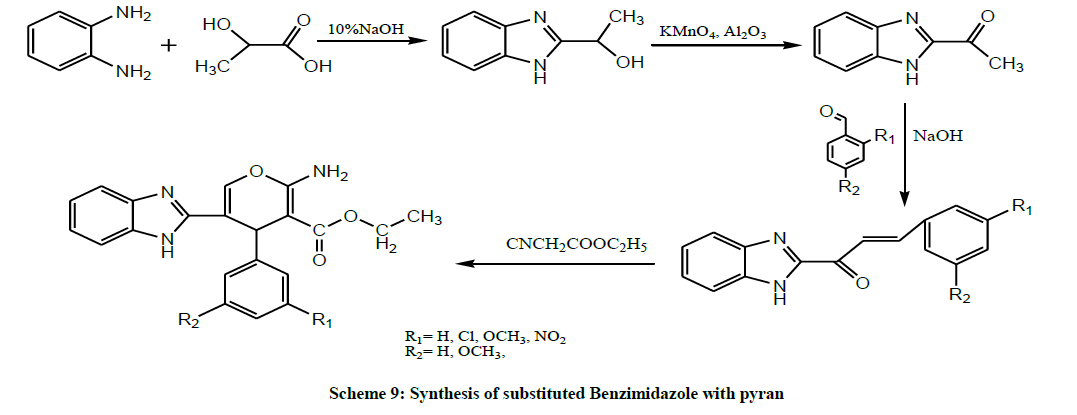
Year 2011 seemed to be a boon for medicinal chemistry as several active Benzimidazolyl chalcones was found. A series of substituted Benzimidazole with pyran were synthesized (Scheme 9) and screened for their antitubercular activity by Divakar et al. All the synthesized compounds were found to be active against Mycobacterium tuberculosis. The 2-methoxy phenyl derivative exhibited better anti tubercular activity compared to the 4-methoxy phenyl compound [23].
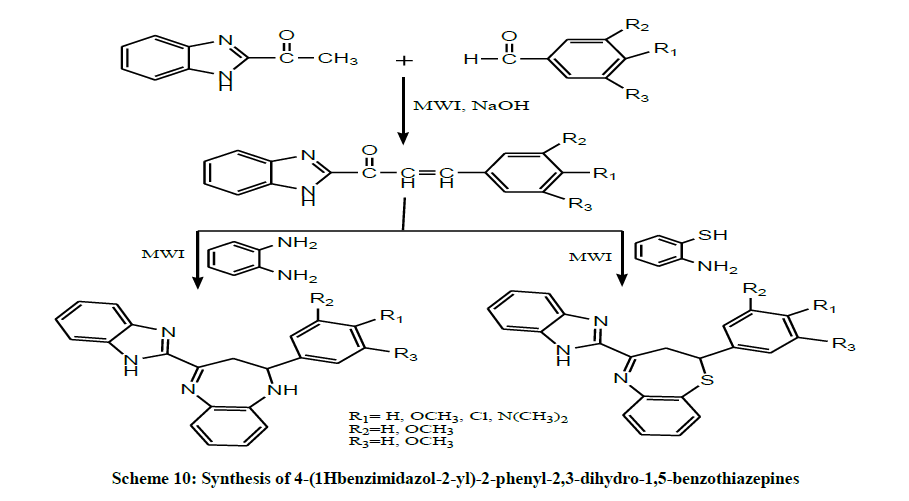
Srivastava et al. in the same year, prepared Chalcones by both conventional and MWI method and were treated with ortho phenylenediamine to yield 4-(1H-benzimidazol-2-yl)-2-phenyl-2,3-dihydro-1H-1,5- benzodiazepines. The chalcones on treatment with 2-amino thiophenol offered 4- (1Hbenzimidazol-2-yl)-2-phenyl-2,3-dihydro-1,5-benzothiazepines (Scheme 10). The compounds were screened for their antimicrobial activity in vitro. The results obtained from this study confirmed that the product formed under microwave irradiation were better in yield and purity [24].
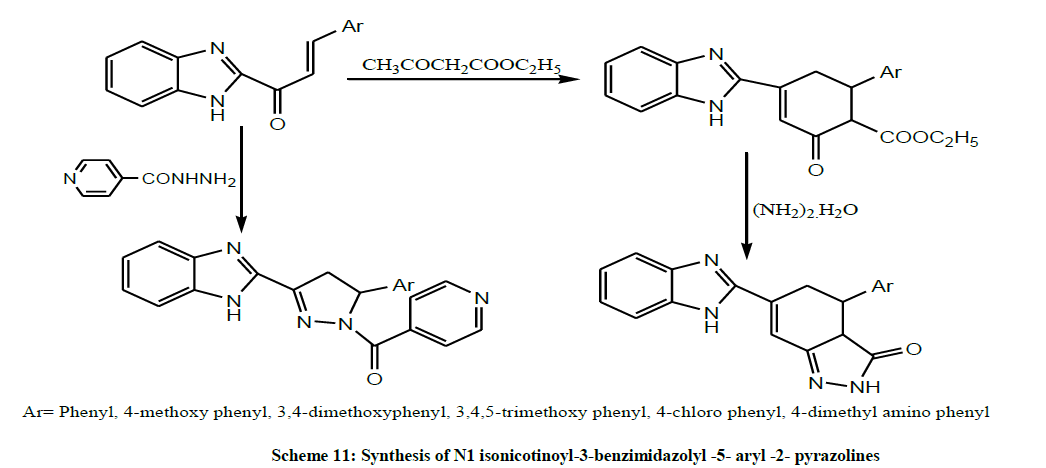
Later, Srivastava et al. treated Benzimidazolyl Chalcones with isonicotinoyl hydrazide (INH) to afford N1 isonicotinoyl-3-benzimidazolyl -5- aryl -2- pyrazolines by both microwaves assisted and conventional heating methods (Scheme 11). The synthesized compounds were screened for their antimicrobial activity in vitro [25].
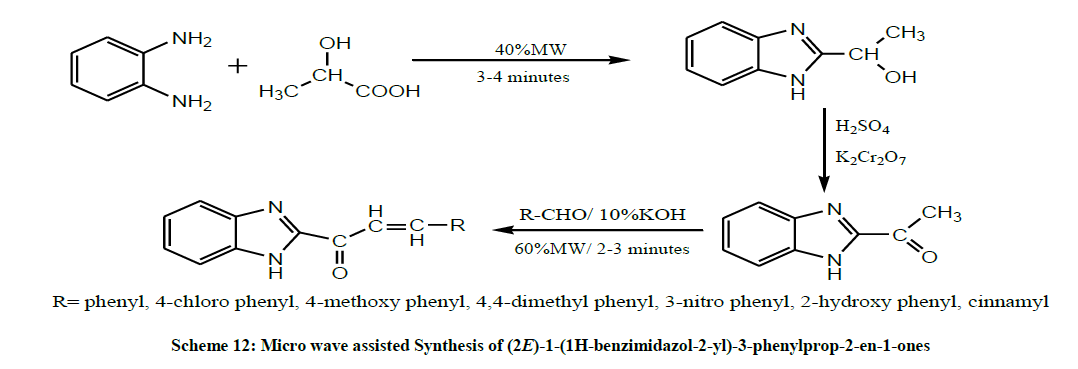
In the same year, Mathew et al. performed the micro wave assisted Synthesis of (2E)-1-(1H-benzimidazol-2-yl)-3-phenylprop-2-en-1-ones by reacting 2-acetyl-Benzimidazole with appropriate aldehydes in the presence of a base (Scheme 12). All the synthetic derivatives were evaluated for their antimicrobial studies. Most of the derivatives were showed good activity towards Gram-positive bacteria and less activity towards Gram-negative bacteria [26].
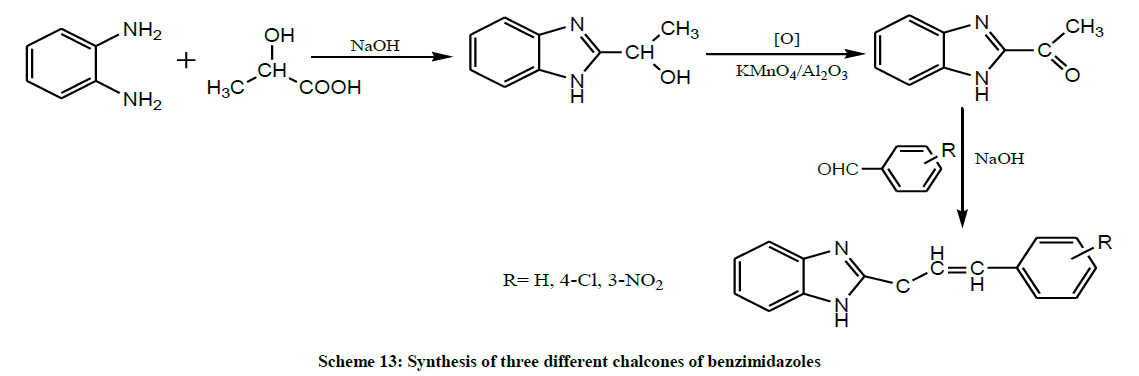
Meena Chandran (2011) synthesized three different chalcones of benzimidazoles from o-phenylenediamine and lactic acid as starting materials, whereas 2-(ahydroxy ethyl) benzimidazole and 2- Acetyl benzimidazole as intermediates (Scheme 13). Then the synthesized compounds were checked for their anticholesterol effect by molecular docking studies against HMG CoA Reductase. Out of the 3 derivatives, one shows highest affinity towards HMG CoA Reductase protein compared with that of the other 2 compounds [27].
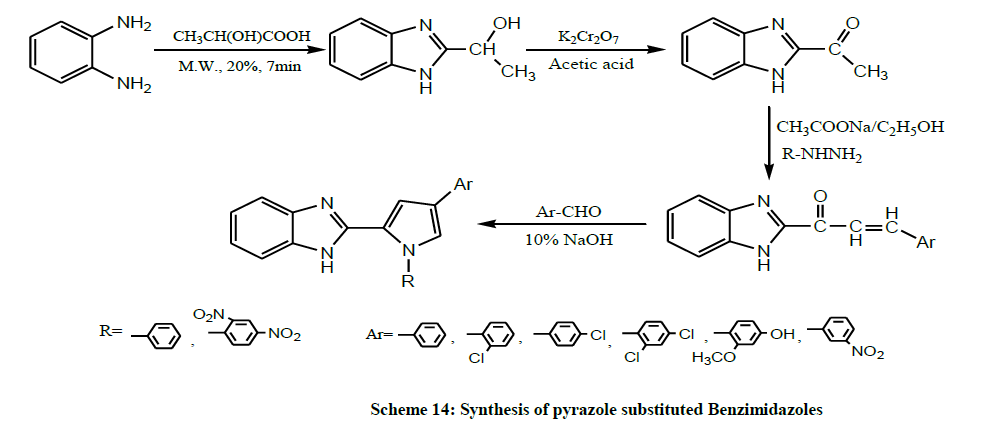
A series of novel and significant compounds containing pyrazole substituted Benzimidazoles (Scheme 14) were synthesized from o-phenylenediamine under microwave irradiation by Kalirajan et al. All the synthesized compounds were evaluated for their anti-bacterial, antifungal, anticancer and anti-tubercular activities [28].
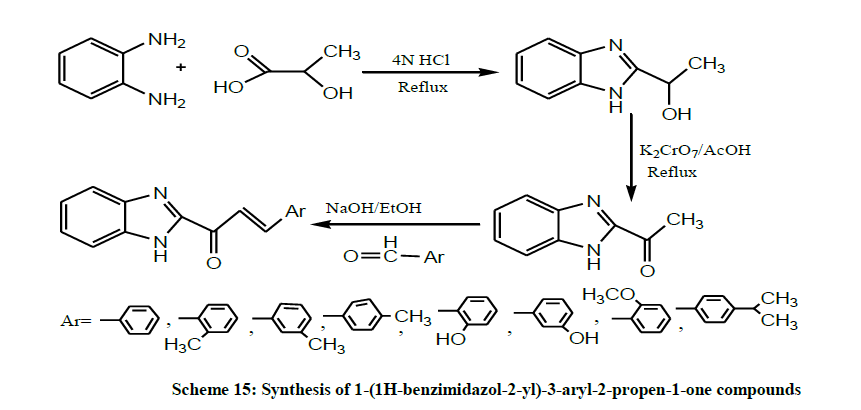
Later, A series of 1-(1H-benzimidazol-2-yl)-3-aryl-2-propen-1-one compounds (Scheme 15) was synthesized by condensation reaction of 2- acetylbenzimidazole with aryl and heteroaryl aldehydes derivatives by Ouattara et al. All compounds were screened in vitro for their nematicidal activity. Preliminary structure-activity relationship show that replacement of phenyl group in position 1 of 1,3-diphenyl-2-propen-1-one by Benzimidazole ring induces the occurrence of a potential anthelmintic activity. So the hybrid benzimidazolyl-chalcones are therefore promising candidates for the development of new anthelmintic agents substituted at positions 5 and/or 6 of the Benzimidazole ring [29].
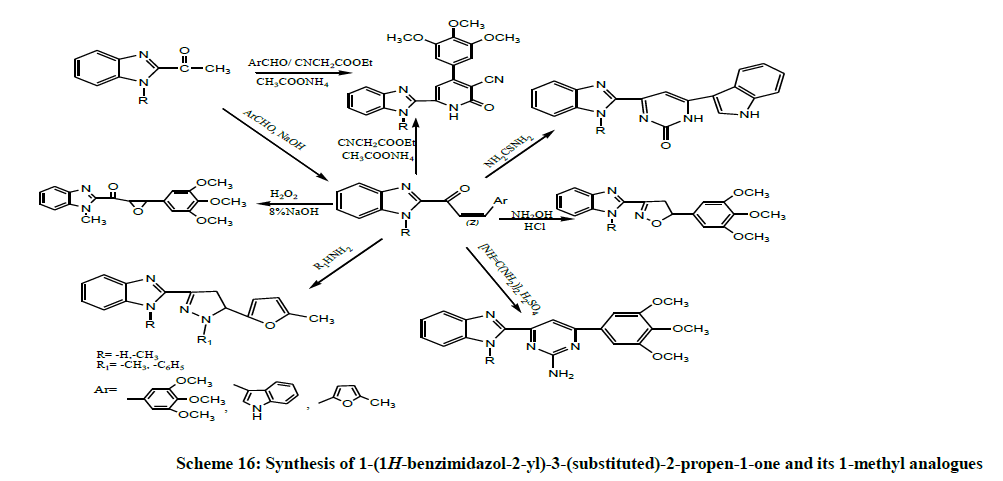
Zienab et al. (2011) synthesized a series of 1-(1H-benzimidazol-2-yl)-3-(substituted)-2-propen-1-one and its 1-methyl analogues and further cyclized with different reagents such as ethyl cyanoacetate, thiourea, hydroxylamine hydrochloride, guanidinium sulfate, methylhydrazine, phenylhydrazine and/or hydrogen peroxide in different reactions to produce pyridones, pyrimidinethione, isoxazole, aminopyrimidine, pyrazoline and epoxy derivative respectively (Scheme 16). The anticancer activity of some of the newly synthesized compounds was evaluated against HEPG2 (human liver carcinoma cell line) and PC12 (pheochromocytoma of the rat adrenal medulla) cells. Benzimidazole-2-isoxazole derivative exhibited high potency against HEPG2 and PC12 cells [30].
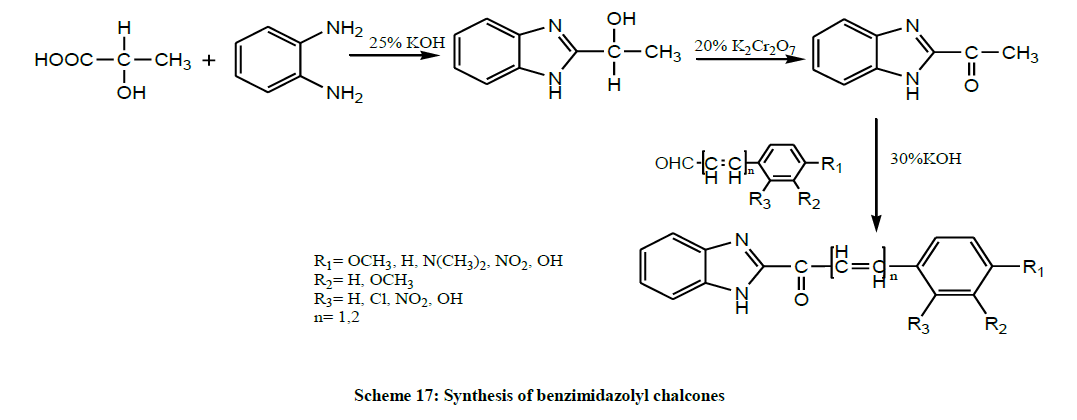
A series of 2-substituted benzimidazolyl chalcones (Scheme 17) were synthesized by condensation reaction of 1-(1H-benzoimidazol-2- yl)ethanone with various substituted aromatic aldehydes in presence of mild alkali by Agarawal and Selvakumar 2011. All the synthesized benzimidazolyl chalcones showed moderate to appreciable significant analgesic activity and also some of the compounds exhibited significant good anti-inflammatory properties [31].
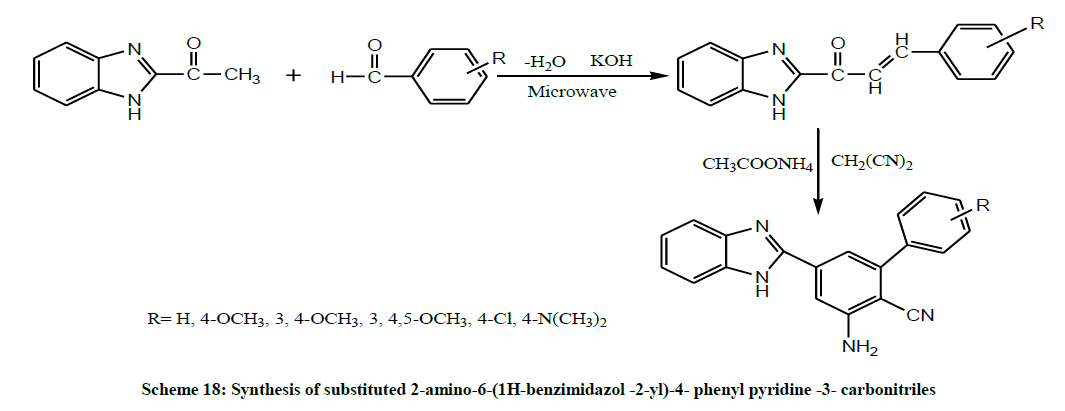
Later, Janardan et al. reacted 2-Acetyl Benzimidazoles with substituted benzaldehydes in presence of ethanol to furnish substituted chalcones. These chalcones were further treated with Malononitrile & Ammonium Acetate to afford substituted 2-amino-6-(1H-benzimidazol -2-yl)-4- phenyl pyridine -3- carbonitriles (Scheme 18). All the compounds were screened for their antibacterial and antifungal activity. The compounds exhibited good antibacterial and moderate antifungal activities [32].
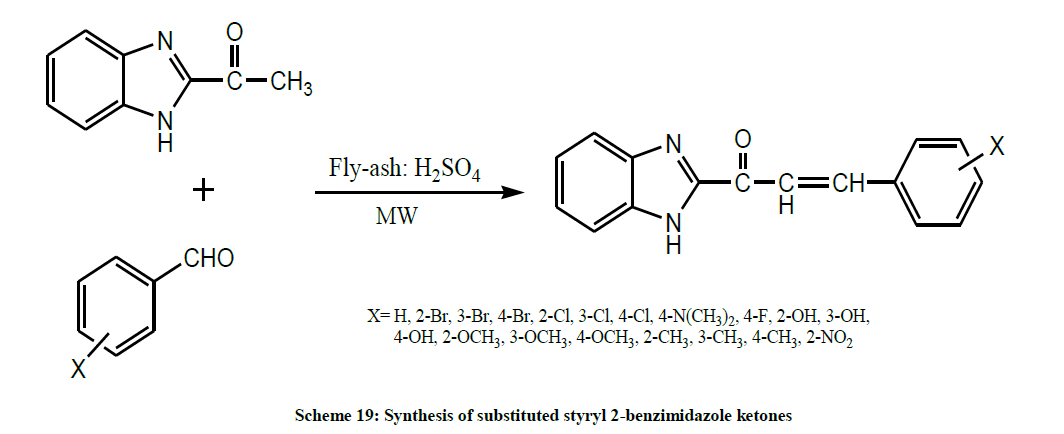
Janaki et al. in 2012 synthesized some new substituted styryl 2-benzimidazole ketones (Scheme 19) by fly-ash: H2SO4 catalyzed aldol condensation of 2-benzimidazole methyl ketone and various substituted benzaldehydes in microwave oven [33].
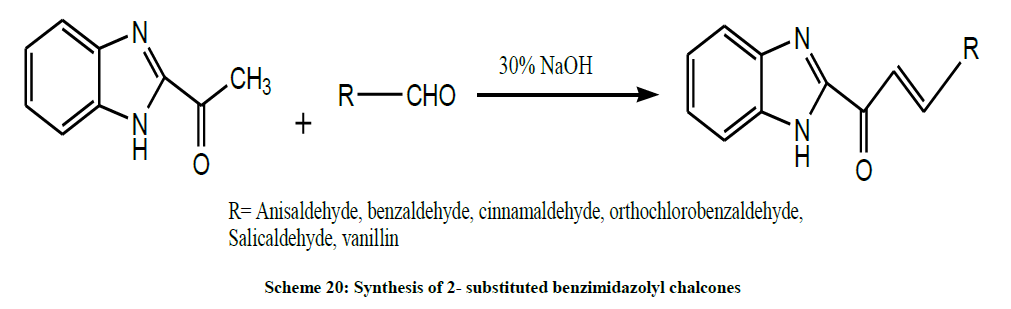
A series of 2- substituted benzimidazolyl chalcones (Scheme 20) were synthesized by condensation reactions of 2-acetyl Benzimidazole with various substituted aromatic aldehydes in presence of alkali by Selakumar et al. All the synthesized chalcones exhibited moderate to good significant analgesic activity and showed appreciable anti-inflammatory activity. The tested chalcones has depicted good central nervous system depressant properties [34].
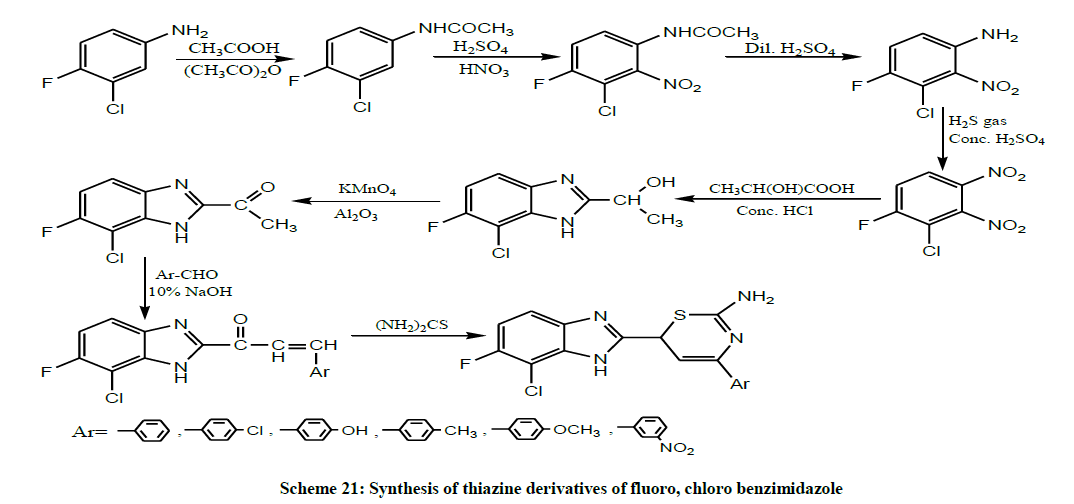
In the same year, Ramjith et al. prepared the chalcone derivatives by the condensation of fluoro, chloro-2- acetyl benzimidazole with different aldehydes and the resulting compounds were cyclized with thiourea, to get the thiazine derivatives of fluoro, chloro benzimidazole (Scheme 21). The synthesized compounds were screened for their antibacterial and analgesic activity. Compounds containing electron withdrawing groups in the substituted benzimidazole thiazine were found to show potent analgesic and antibacterial activities [35].
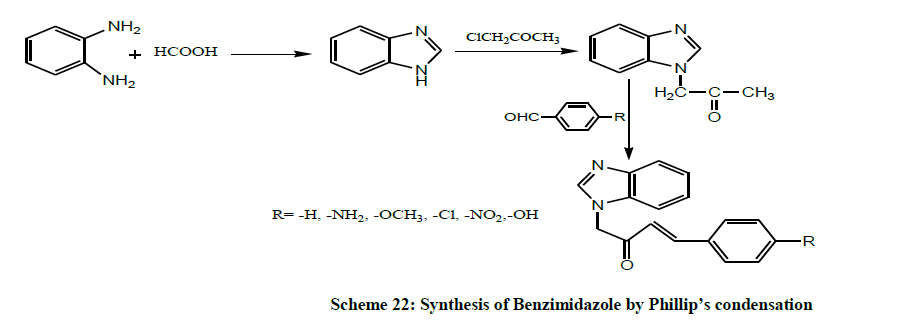
In year 2013, Sridevi et al. synthesized Benzimidazole by condensing o-phenylene diamine with carboxylic acid by using Phillip’s condensation. It was then acetylated using chloroacetone. The formed acetylated product was subjected to aldol condensation with different aromatic aldehydes to give six different novel benzimidazole chalcones (Scheme 22). All the synthesized compounds were screened for their antibacterial activity [36].
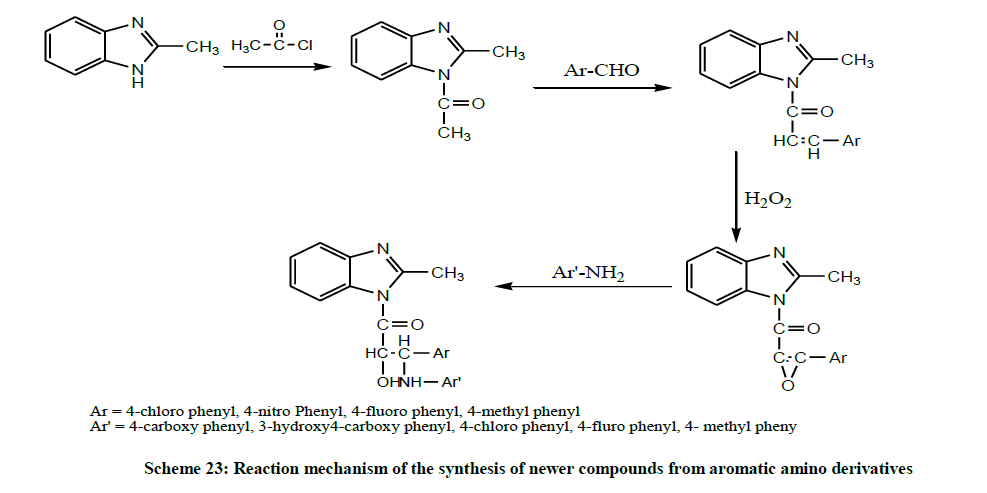
In the same year, Umma et al. synthesized a series of 4-[1-(4-substituted phenyl)-2-hydroxy- 3-(2-methyl-benzimidazole-1-yl) - 3-oxo- propyl amino] substituted aromatic amino derivatives by acetylating 2-methyl benzimidazole and further chalcones were prepared in alkaline medium using aromatic aldehydes. The chalconic compound was treated with glacial acetic acid and hydrogen peroxide for preparing 1- {[3-(4- substituted phenyl) oxiran-2yl] carbonyl} 2-methyl-1H-benzimidazole. Then it was treated with different substituted amines to synthesize the newer compounds (Scheme 23). The synthesized compounds were evaluated for their antifungal potentials activity. According to the SAR, the introduction of chlorophenyl and fluorophenyl substituents with 2-methyl benimidazole moiety has a major role in producing significant antifungal potentials [37].
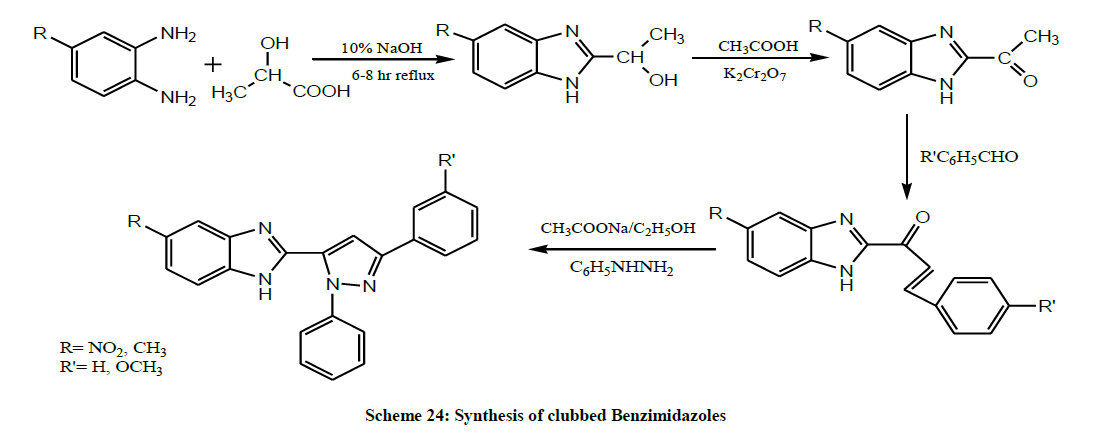
Patel et al. in 2014 synthesized clubbed Benzimidazoles substituted with pyrazole i.e 2-(1,3-diphenyl-1H-pyrazol-5-yl)-1H-benzo[d]imidazole by cyclization of Benzimidazolyl chalcones with hydrazine hydrate (Scheme 24). All the synthesized derivatives were evaluated for their anti-tubercular activity [38].
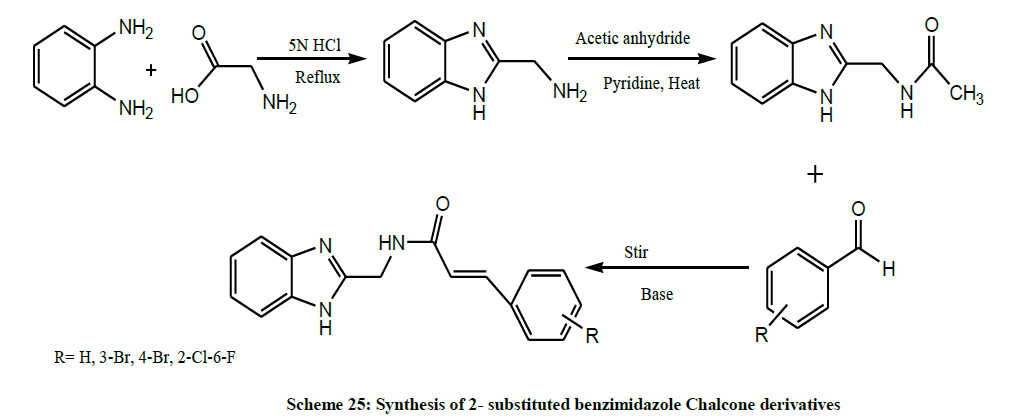
A series of 2- substituted benzimidazole Chalcone derivatives (Scheme 25) were synthesized by Rajput et al. All these synthesized compounds were tested for their Antifungal activity against C. albicans in polar solvent DMSO. The docking and antifungal screening showed that compound containing bromine group as substituent exhibited best receptor binding (as indicated by best docking score) as well as comparable antifungal activity [39].
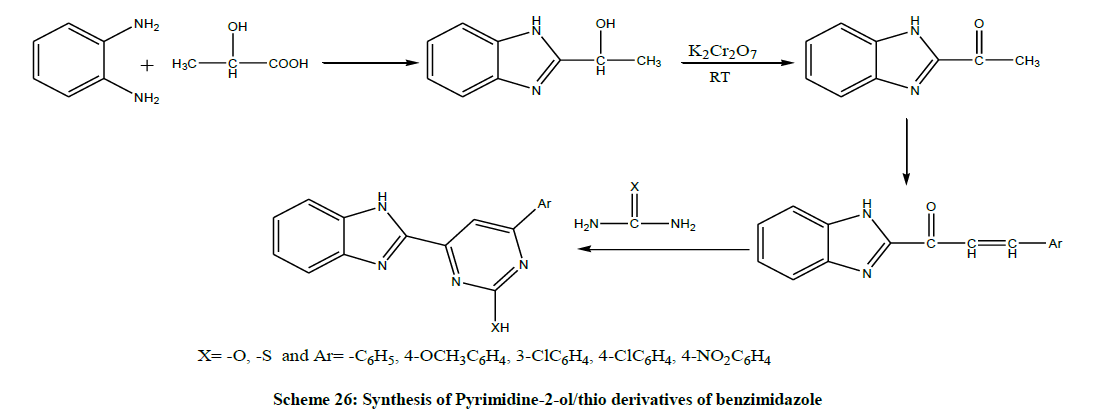
In the same year, Rajawat et al. investigated the comparative study between traditional (conventional) and microwave technique of metal complexes of Bi (III) with pyrimidine derivatives as a ligand and also determining their antimicrobial activity against selected organisms. Pyrimidine-2-ol/thio derivatives of benzimidazole have been synthesized by 2-acetyl benzimidazole with different aromatic aldehydes and finally the product was cyclised with urea and thiourea to form pyrimidine derivatives of benzimidazoles by using both the microwave technique as well as conventional technique (Scheme 26) [40].
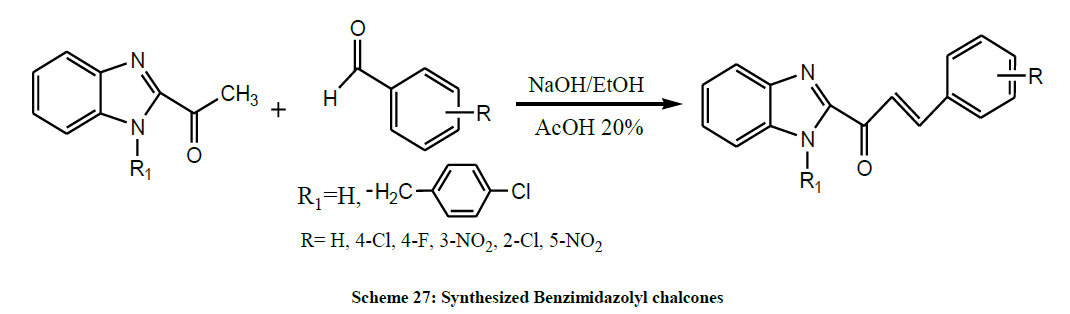
Later, Sissouma et al. synthesized Benzimidazolyl chalcones by reaction of various aromatic aldehydes with N-(4-chlorobenzyl)-2- acetylbenzimidazole and its precursor 2-acetylbenzimidazole (Scheme 27). These synthesized compounds were evaluated for their antifungal activities against a clinical strain of Candida albicans in order to determine the minimum inhibiting quantity (MIQ). This screening showed that some compounds had anti-Candida efficiencies (MIQ = 5, 1.25 and 0.625 μg) higher than those of chlormidazole (MIQ = 10 μg) [41].
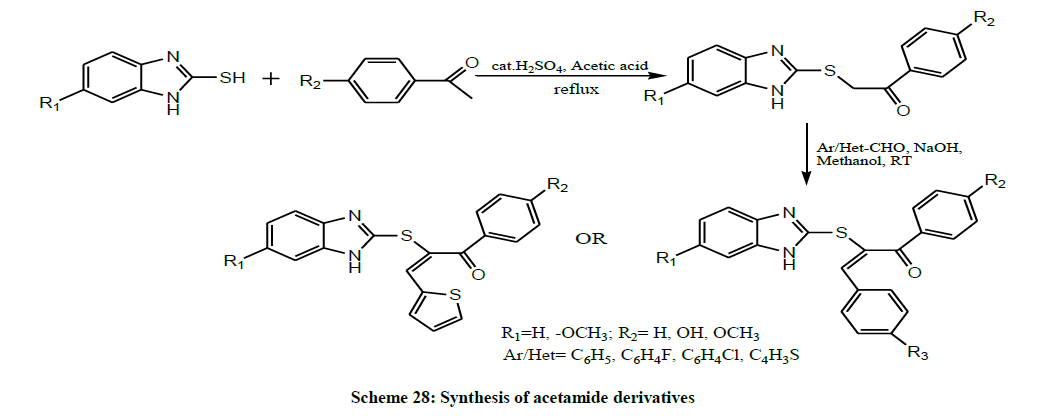
Gadhiya et al. in 2016 synthesized 2-(1H-benzo[d]imidazol-2-ylthio)-3-(subsitutedphenyl)-1-(subsitutedphenyl) prop-2-en-1-one and N-(3- chlorophenyl)-2-(1-(2-hydrazinyl-2-oxoethyl)-1H-benzo[d] imidazol-2-ylthio) acetamide derivatives (Scheme 28). All the synthesized compounds were subjected for their antimicrobial evaluation [11].
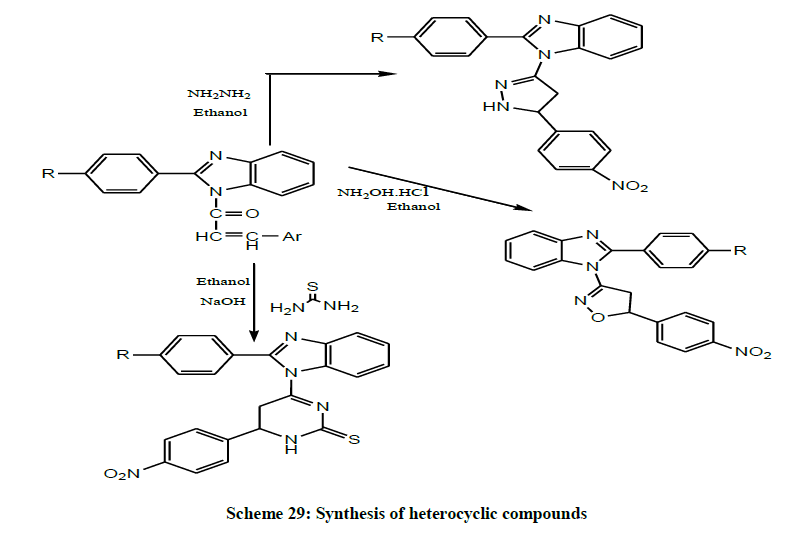
In the same year, Mohamad et al. prepared a number of benzimidazole derivatives via the condensation reaction of o-phenylene diamine with various aromatic carboxylic acids in the presence of hydrochloric acid. The acetylated benzimidazoles then allowed reacting with benzaldehyde derivatives to give chalcones. In order to achieve the final heterocyclic compounds those chalcones were treated with hydroxylamine hydrochloride, hydrazine hydrate and thiourea through Claisen-Schmidt condensation (Scheme 29). It is noteworthy to mention that the pyrazolo, isoxazolines, and pyrimidines derivatives having with variety of substituents such as chloro, nitro were prepared in good yield [42].
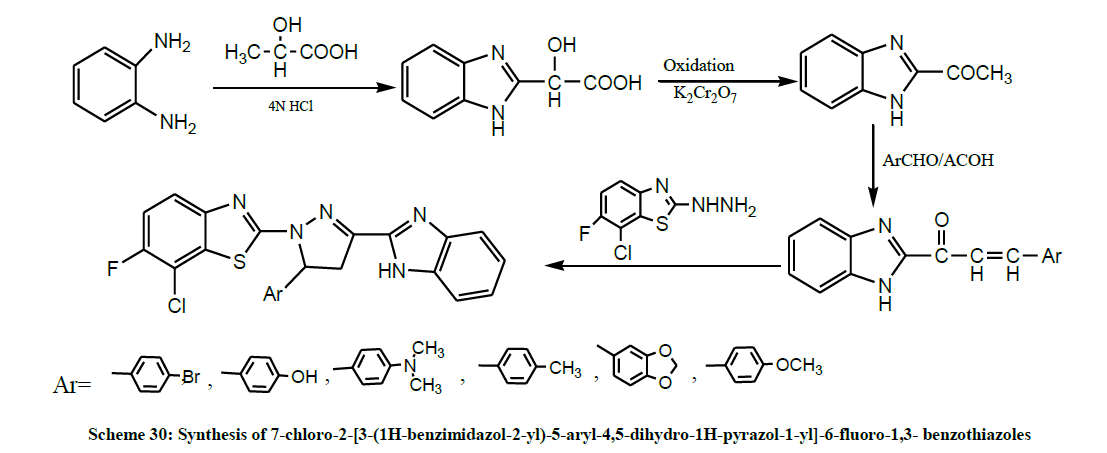
A series of 7-chloro-2-[3-(1H-benzimidazol-2-yl)-5-aryl-4,5-dihydro-1H-pyrazol-1-yl]-6-fluoro-1,3- benzothiazoles (Scheme 30) were synthesized by Sharmila et al. All the synthesized compounds were screened for anti-inflammatory and analgesic activity. The results reveal that compounds show moderate to significant anti-inflammatory and analgesic activity [43].
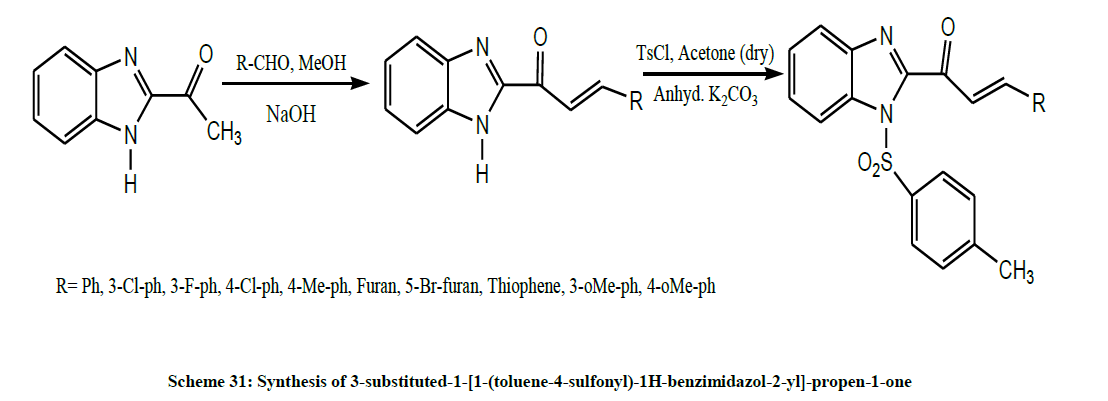
Later, Meshram et al. synthesized a series of 3-substituted-1-[1-(toluene-4-sulfonyl)-1H-benzimidazol-2-yl]-propen-1-one (Scheme 31) by tosylation of Benzimidazole chalcones using ultrasound in lesser time with higher yields. The results of biological study show that introduction of tosyl group in Benzimidazole chalcones enhances the inhibition of glycosidases and microorganism in comparison to parent compounds [44].
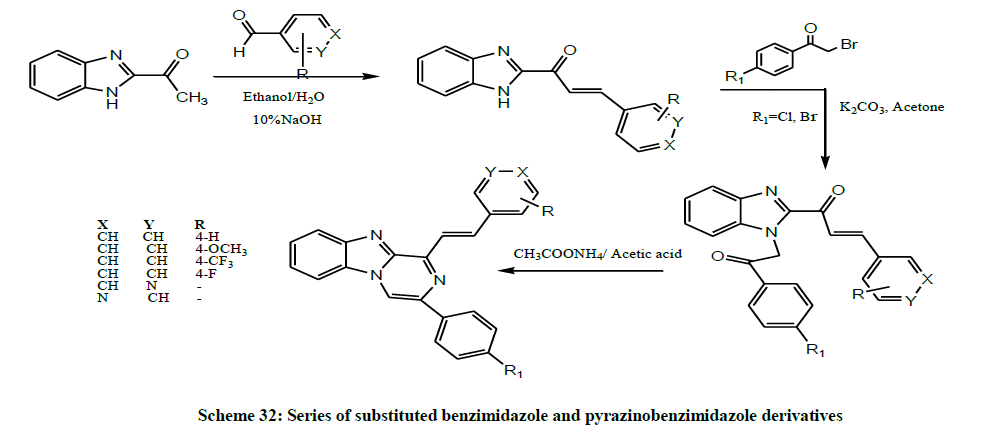
Mohamed et al. in 2017 developed a series of substituted benzimidazole and pyrazinobenzimidazole derivatives (Scheme 32) and evaluated the cytotoxic activity of these designed compounds. Among the tested compounds, some compounds showed the highest and broad spectrum activity against the three cancer cell lines. Molecular docking studies confirmed the strong cytotoxic activity of these compounds over MCF-7 and the postulation that these active compounds may act on the same enzyme target where EGFR inhibitor, Erlotinib, acts [45].
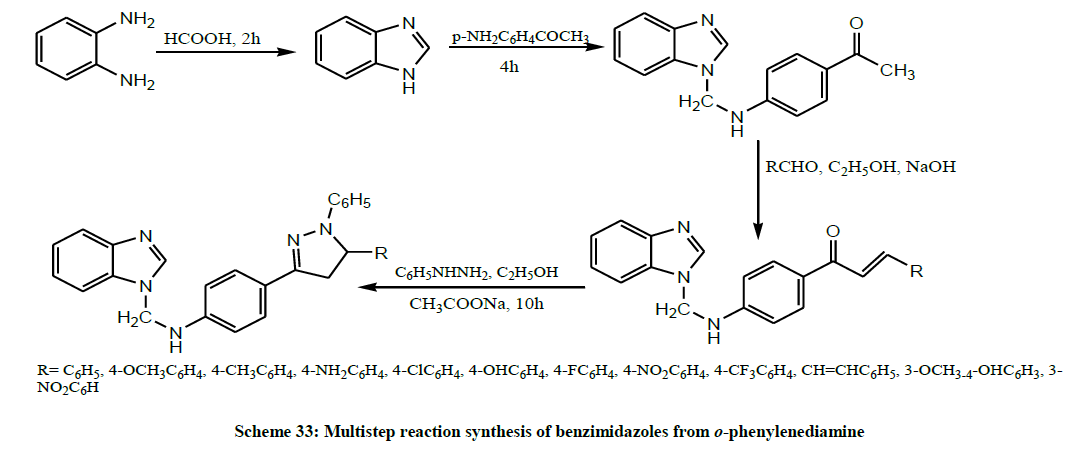
A series of novel pyrazole attached benzimidazoles (Scheme 33) were synthesized from o-phenylenediamine by the multistep reaction synthesis by Krishnanjaneyulu et al. All the title compounds were screened for their in vitro antimicrobial activity. Results revealed that compounds containing an electron-withdrawing group at the phenyl group attached to C-5 of pyrazole displayed superior antimicrobial activities than compounds possessing an electron-releasing group [46].
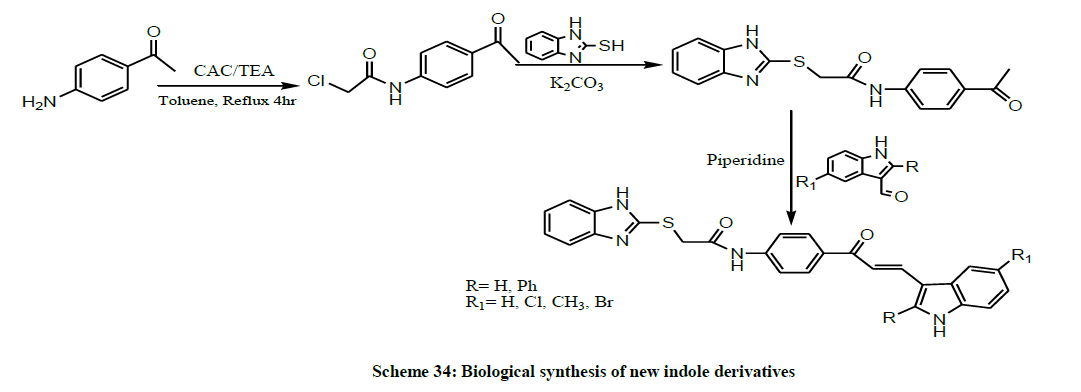
In the same year, Biradar et al. synthesized biologically active new indole derivatives coupled with benzimidazole viz., 2-((1Hbenzo[d]imidazol- 2-yl) thio)-N-(4-(3-(1H-indol-3-yl) acryloyl) phenyl) acetamide (Scheme 34). All these newly synthesized compounds were screened for their in vitro antimicrobial and antioxidant activities [47].
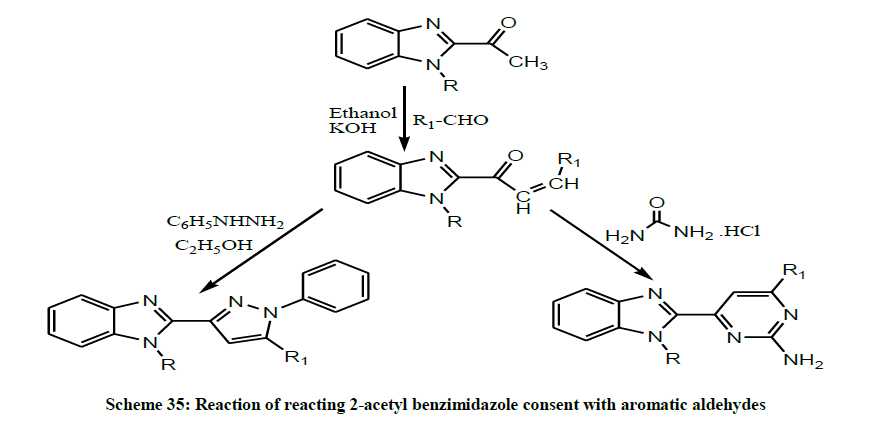
Later, A series of novel benzimidazole derivatives (Scheme 35) were synthesized by reacting 2-acetyl benzimidazole consent with various substituted aromatic aldehydes to obtain preferred intermediate chalcones, then these intermediates were undergo the cyclo-condensation with guanidine hydrochloride and phenyl hydrazine yielded benzimidazole derivatives by Rajendran et al. The synthesized compounds were screened for their in vitro anticancer activities. Among them the compounds containing benzimidazole with pyrimidines moiety and compounds containing benzimidazole with pyrazole moiety showed significant anticancer activity (Table 1) [48].
| S. No. | Authors | Structure | Pharmacological activity |
|---|---|---|---|
| 1 | Krishnanjaneyulu et al.2014 | 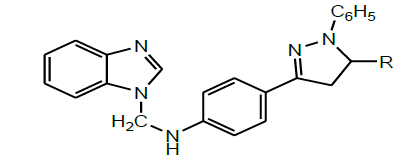 |
Antimicrobial activity [46] |
| 2 | Abd and Soliman, 2011 | 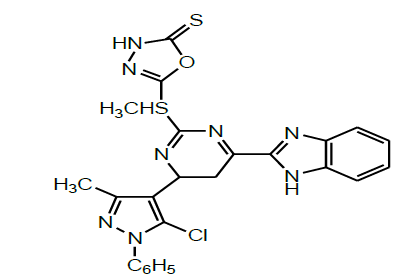 |
Cytotoxic and antioxidant activities [49] |
| 3 | Ouattara et al. 2011 | 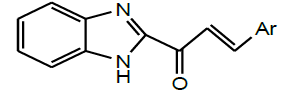 |
Anthetmintic activity [30] |
| 4 | Rajput et al.2015 | 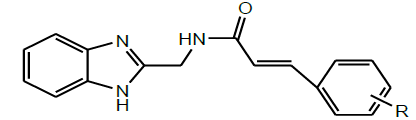 |
Antifungal activity [39] |
| 5 | Sridevi et al.2017 | 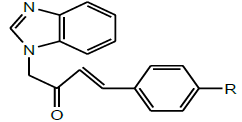 |
Antibacterial activity [37] |
| 6 | Agarawal et al.2011 | 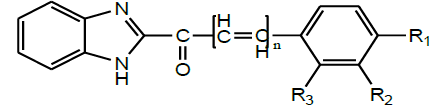 |
Analgesic, anti-inflammatory and CNS activities [32] |
| 7 | Srivastava et al.2009 | 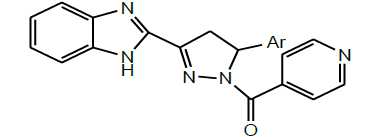 |
Antimicrobial activity [26] |
| 8 | Mohamed et al.2017 | 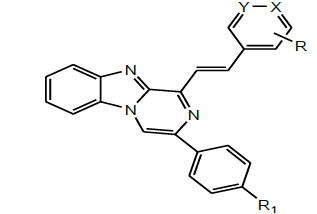 |
Antitumor activity [42] |
| 9 | Sissoumaet al.2015 | 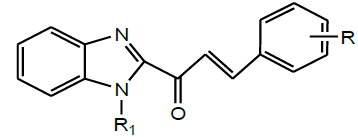 |
Antifungal activity [41] |
| 10 | Bahera et al.2016 | 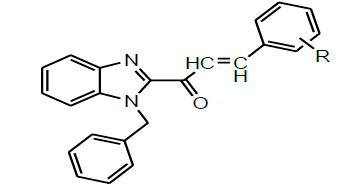 |
Antibacterial activity [50] |
| 11 | Biradar et al.2017 | 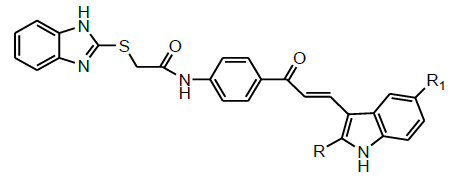 |
Antioxidant activity [47] |
| 12 | Divakar et al. 2011 | 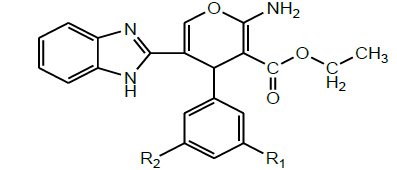 |
Anti-tubercular activity [24] |
| 13 | Janaki et al. 2016 | 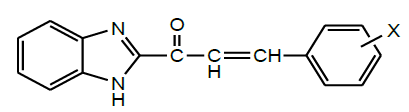 |
insect anti-feedant activities [34] |
| 14 | Kalirajan et al. 2011 | 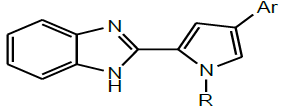 |
Anticancer activity [29] |
| 15 | Meshram et al. 2016 | 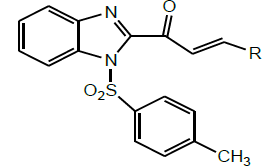 |
Glucosidase inhibitor [44] |
| 16 | Ramjith et al. 2012 | 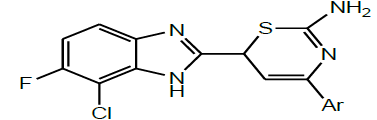 |
Antibacterial, Analgesic activities [36] |
| 17 | Umaa et al. 2013 | 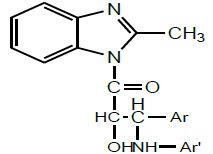 |
Antifungal activity [38] |
| 18 | Zienab et al. 2011 | 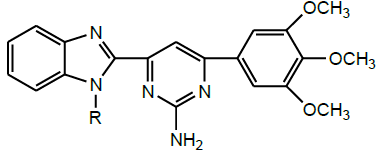 |
Anticancer activity [31] |
Table 1: Pharmacological activities of Benzimidazolyl chalcone derivatives
Conclusion
The Benzimidazolyl chalcones exhibit diverse pharmacological activities. The diversity of benzimidazole derivatives are utilized in many therapeutic applications such as anti-cancer, antimicrobial, antitubercular, antioxidant, analgesic and anti-inflammatory activity. This review paper compiled the efficient and economical synthetic methods of Benzimidazolyl chalcones. These synthetic schemes become very useful for chemists and workers in this field, to develop protocols for the large production of new derivatives of Benzimidazolyl chalcones.
Acknowledgement
The authors are thankful to the Director (Dr. /Prof. Divya Juyal) of the Department of Pharmaceutical Sciences of Himalayan institute of Pharmacy and Pharmacy for providing necessary support to carry out the work.
References
- A.K. Mishra, V. Gautam, A. gupta, R. Bansal, P. Bansal, S. Kumar, V. Gupta, J. Pharm. Res., 2010, 3(2), 371-378.
- L.E. Ouasif, A. Bouyahya, R. Zniber, M.E. Ghoul, R. Achour, H. Chakchak, A. Talbaoui, H.E. Boury, N. Dakka, Y. Bakri, Mediterranean J. Chem., 2017, 6(3), 77-87.
- Salahuddin, M. Shaharyar, A. Mazumdar, M.M. Abdullah, Arabian J. Chem., 2017, 10, 503-508.
- G. B. Patel, R.R. Mahire, D.H. More, Der Pharma Chemica., 2013, 5(6), 369-376.
- M. Shaharyar, A. Mazumdar, Salahuddin, R. Garg, R.D. Pandey, Arabian J. Chem., 2016, 9, 342-347.
- P.M. Prasad, S. Raja, European J. Biomed. Pharma. Sci., 2017, 4(6), 617-626.
- M.M. Kandeel, S.M. Ali, M.A. Abdelgawad, M.S. Abdel-Bakky, F.E. Mohamed, Der Pharma Chemica., 2016, 8(1), 117-123.
- R.S. Kankate, P.S. Gide, D.P. Belsare, Der Pharma Chemica., 2014, 6(6), 396-405.
- H.M.T. Albuquerque, C.M. M. Santos, J.A.S. Cavaleiro, A.M.S. Silva, Current Org. Chem., 2014, 18, 1-26.
- A.R. Semwal, K.L. Dangwal, Int. J. Sci. Res., 2015, 4(6), 2972-2974.
- B.D. Gadhiya, M.R. Rajput, A.H. Bapodra, K.D. Ladva, Der Pharma Chemica., 2016, 8(16), 40-47.
- A. Pareek, P. Rani, N. Kumar, P. Sharma, D. Kishore, Int. J. Chem. Pharma. Sci., 2013, 4(3), 19-24.
- A.M. Asiri, H.M. Marwani, K.A. Alamry, M.S. Al-Amoudi, S.A. Khan, S.A. El-Daly, Int. J. Electrochem. Sci., 2014, 9, 799-809.
- G.I. Jimenez, J.A. Bravo, J.L. Vila, Bolivian J. Chem., 2016, 33(5), 179-182.
- P.K. Dubey, C.R. Kumar, B. Babu, Indian J. Chem., 2003, 42B, 3128-3130.
- G. Roman, I. Manciulea, L. Dumitrescu, Indian J. Het. Chem., 2004, 51, 1-2.
- Y.M. Shahar, M.M. Abdullah, J. Majeed, World Aca. Sci. Engi. Tech., 2009, 31, 589-595.
- J. Rajora, Y.K. Srivastava, Rasayan J. Chem., 2009, 2(3), 655-658.
- B.A. Baviskar, B. Baviskar, M.R. Shiradkar, U.A. Deokate, S.S. Khadabadi. E. J. Chem., 2009, 6(1), 196-200.
- J.S. Yadav, Y.K. Srivastava, Rasayan J. Chem., 2010, 3(4), 726-730.
- Y.K. Srivastava, J. Rajora, J. Yadav, R. Kumar, Indian J. Chem., 2010, 49B, 989-993.
- B.M. Sahoo, T.P. Bahera, R. Kumar, Int. J. Chem. Tech. Res., 2010, 2(3), 1634-1637.
- F.M. Saleshier, S. Singh, J. Karim, M.C. Divakar, Eur. J. Exp. Bio., 2011, 1(2), 150-159.
- J.S. Yadav, J. Rajora, Y.K. Srivastava, Arch. Applied Sci. Res., 2011, 3(2), 192-198.
- J.S. Yadav, Y.K. Srivastava, Der Pharmacia Lettre., 2011, 3(2), 284-291.
- B. Mathew, G. Unnikirishnan, V.P. Shafeer, M. Mohammed, P. Femina, Der. Pharma. Chemica., 2011, 3(6), 627-631.
- M. Chandran, J. Pharm. Res., 2012, 5(1), 324-326.
- R. Kalirajan, L. Rathore, S. Jubie, B. Gowramma, S. Gomathy, S. Sankar, Indian J. Chem., 2011, 50B, 1794-1799.
- M. Ouattara, D. Sissouma, M.W. Kone, H.E. Menen, S.A. Toure, L. Ouattara, Tropical J. Pharma. Res., 2011, 10(6), 767-775.
- Z.M. Nofal, E.A. Soliman, A.E. Karim, E. Zahar, A.M. Srour, S. Sethumadhavan, T.J. Maher, Acta Poloniae Pharma-Drug Res., 2011, 68(4), 519-534.
- S. Selvakumar, P.C. Aggarwal, Int. J. Pharm. Ind. Res., 2011, 1(4), 354-366.
- J.S. Yadav, Y.K. Srivastava, Der Chemica Sinica., 2011, 2(1), 1-7.
- P. Janaki, K.G. Sekar, G. Thirunarayanan, J. Saudi Chem. Soc., 2016, 20, 58-68.
- S. Selvakumar, I.S. Babu, N. Chidambaranathan, Int. J. Phyto Pharmacol., 2012, 3(2), 163-172.
- G. Banda, S.M. Hipparagi, U.S. Ramjith, C.M. Jacob, Int. J. Res. Pharmacy Sci., 2012, 2(3), 146-158.
- C. Sridevi, M.M. Kannan, G. Abhinayani, N. Sravya, Chem. Sci. Trans., 2013, 2(3), 922-926.
- K. Umaa, K.K. Kumar, B.D. Kumar, K. Kannan, Int. J. Chem. Sci. Tech., 2013, 3(1), 9-14.
- Y. Patel, P.M. Sabale, A. Rathod, A. Nagar, V. Patel, A. Patel, Pharmagene., 2014, 2(1), 22-26.
- P.B. Rajput, S.A. Phadke, P.V. Bandiwadekar, Int. J. Pharma. Chem. Bio. Sci., 2015, 5(3), 712-718.
- A.M. Chaturvedi, Y.K. Mishra, V. Rajawat, American J. Phytomed. Clinical Ther., 2015, 3(4), 383-393.
- D. Sissouma, M. Ouattara, D. Zon, M.W. Kone, African J. Pharm. Pharmacol., 2015, 9(12), 418-423.
- M.F. Ali, A.M. Khlafulla, R. Mohamed, Int. J. Eng. Applied Sci., 2016, 3(6), 13-16.
- S.A. Gote, S.B. Kumar, E.L. Gaviraj, Der Pharma Chemica., 2016, 8(5), 33-37.
- G.A. Meshram, V.A. Vala, P.A. Wagh, S.S. Deshpande, Indian J. Chem., 2016, 55B, 613-623.
- A.A.B. Mohamed, F.A. Badria, A.R. Maarouf, N.I. Abdel-Aziz, F. Elsenduny, A.M.M. Abdel-Aziz, A.M. Bayomi, J. Applied Pharma. Sci., 2017, 7(6), 206-214.
- I.S. Krishnanjaneyulu, G. Saravanan, J. Vamsi, S. Pamidipamula, U.B. Jarugula, M.V.S. Kumar, J. Adv. Pharm. Tech. Res., 2014, 5(1), 21-27.
- B.S. Naraboli, S. Biradar, Int. J. Pharm. Pharma. Sci., 2017, 9(8), 128-138.
- S.S. Rajendran, G. Geetha, R. Venkatanarayanan, N. Santhi, Int. J. Pharma. Sci. Res., 2017, 8(7), 3014-3024.
- S.N. Abd el al, F.M.A. Soliman, Der Pharma Chemica., 2015, 7(4), 71-84.
- G.K. Padhy, J. Panda, A.K. Bahera, Der Pharma Chemica., 2016, 8(13), 235-241.