Review Article - Der Pharma Chemica ( 2017) Volume 9, Issue 2
A Review on Evolution in Triglyceride Determination
Rekha Dhull1, Vikas Dhull2, Kavita Rathee1 and Sandeep Singh12University Institute of Engineering and Technology, Maharshi Dayanand University, Rohtak-124001, India
Abstract
Triglycerides are a kind of lipids found in many food items such as vegetable oils which are rich sources of fats. These are metabolised by animal’s body easily but their increased concentration is dangerous for humans and can cause a variety of life threatening diseases like atherosclerosis, heart attack, stroke, pancreatitis nephritic syndrome, chronic hepatitis, hyper-lipidemia, liver disease, hypothyroidism and diabetes mellitus. There are many methods for the detection and determination of triglycerides. Traditional methods include chemical, enzymatic, fluorimetric and bioluminescent, chromatographic and HPLC and Flow injection analysis, these methods have certain limitations such as they are complex, require sample preparation due to which they are time consuming and also require trained personals. So, there is a great need of the new methods which can overcome these limitations. Biosensors provide the promising future for the easy and the early detection method. Role of biosensors in determining triglyceride concentration is the main highlight of this article. Lipase is the principle enzyme used in triglyceride determination; it produces glycerol, which is further oxidised enzymatically in several ways with glycerol dehydrogenase, glycerol-3-phosphate dehydrogenase, glycerol phosphate oxidase. Principle of working of biosensor, available supports for immobilisation and their advantages have been discussed. Even the sensitivity and performance of biosensors is being improved by using nano materials as transducer. Use of nanostructures like nanoparticle, nanotubes, nanorods, nanoprobes and their properties, merits over other substrates is also a part of the discussion. Recent research works done on triglyceride biosensor have been compiled in this review.
Keywords
Atherosclerosis, Immobilisation, Lipase, Nanoparticles, Transducer, Triglycerides
Introduction
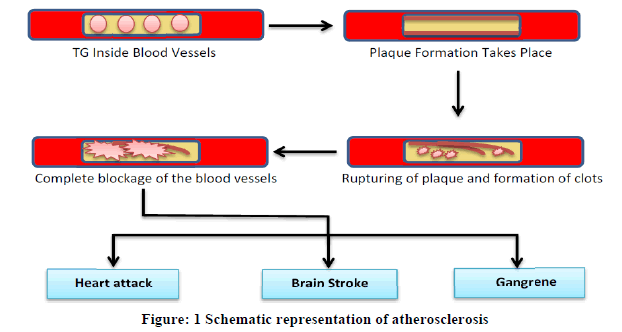
The term triglyceride or triacylglycerol refers to an ester derived from glycerol (trihydroxy alcohol) and three fatty acids. Fatty acid can be palmitic acid, oleic acid, linolenic or linoleic acid or any other saturated or unsaturated fatty acid. This is a major constituent of vegetable oils, animal fats, chicken, eggs, dairy products, beef and pork. High level of Triglyceride (TG) in body enhanced the risk of atherosclerosis. Atherosclerosis leads to human vascular disorders such as coronary artery disease and hardening of the arteries. TGs deposits in arteries and results in plaque formation along the lining of arterial walls and makes the passage narrower which causes hindrance in the blood flow. Sometimes plaque gets ruptured and causes formation of blood clots. Occasionally these clots lead to complete blockage of the blood vessels causing hindrance in blood flow to heart and brain which enhances the potential risk of heart attack and strokes (Figure 1). When blood flow to limbs gets reduced in case of atherosclerosis it causes sores, ulcers or even gangrene. Hypertriglycaemia or elevations in blood triglycerides may also cause inflammation of the pancreas (pancreatitis) [1,2]. Various other disorders like nephritic syndrome, chronic hepatitis, hyperlipidaemia, liver disease, hypothyroidism, diabetes mellitus and alcoholism are also associated with the increased level of TGs (Figure 1) [3,4].
American Heart Association concluded that TG level less than 150 mg/dL (1.7 mmol/L) is normal, level from 150-199 mg /dL (2.25 mmol/L) is borderline-high, level from 200-499 mg/dL (5.65 mmol/L) is high and level above 500 mg/dL (5.65 mmol/L) is very high 5. So, in the above concern there is a requirement of sensitive and accurate method which can detect TG even at lower concentrations at earlier stage. A variety of conventional methods including chemical method (p1 3), enzymatic assays (p1 10), bioluminescent (p1 8), chromatographic (HPLC and Flow injection analysis) (p1 4) methods are available for TG detection but none of them detects the concentration significantly. They also suffer from the drawbacks being time consuming, don’t have specific analytical capability and finally not much accurate so reliable. The above problems associated with the use of conventional methods can be overcome by using biosensors. TG biosensor with different nanomaterials (nanoparticles, nanosheets, nanotubes, Nanorods etc.) and transducers can be fabricated in the laboratory for the onsite screening of the samples [5-10].
Conventional methods for TG determination and their associated drawbacks
The conventional analytical methods for estimation of TG in various samples are dependent upon the difference between the sum concentration of cholesterol, cholesterol and phospholipid against the total concentration of lipids in the particular sample. The conventional methods are categorized among chemical methods, chromatographic methods and mass fragmentation method [11-15].
The chemical methods are subcategorized in titration method, micro analysis method, and automated method. In the titration method the basic principal involved is determining the concentration of unknown sample against the concentration of known sample. In this method the sample is extracted with the help of organic solvents from the crude sample and then subjected to NaOH hydrolysis [16-18]. Then the above obtained sample is again titrated to collect the fatty acids present. The amount of fatty acid recovered is in direct proportion to the amount of TG present in the sample (p1 13). In micro analysis method the samples are screened for the glycerol moieties present on the triglyceride molecule. This method is the direct quantification method of the blood samples. This involves glycerol oxidation reaction with the help of periodate resulting in the colour change and hence the colorimetric detection (p1 14). In the automated method an autoanalyzer is used. The TG is first of all extracted in the solvent and then purified. After this the sample is injected in autoanalyzer in which glycerides are subjected to alkaline hydrolysis at a high temperature [19-22].
The chromatographic methods include Capillary ion exchange column chromatography, thin layer chromatography, high performance liquid chromatography. The capillary ion column gas chromatography method was successfully utilized (p1 19). In this method gas chromatography was directly used for the glycerol detection. The linearity of this method with ion column was 37 m mol/L and with gas chromatography it was found to be 22 m mol/L. In case of thin layer chromatography a stationary phase (alumina, silica, cellulose etc.) is exploited [23-26]. The resulting triglycerides the acid hydrolysis of glycerides resulted in glycerol and finally converted to tri methyl glycerol. The small biological samples were also screened for triglycerides using thin layer chromatography followed by molebdatophosphoric based acid staining (p1 15). The adsorption property along with the refractive index has also resulted in the estimation of triglycerides. A simple method using HPLC has been reported in which the triglycerides were quantitatively determined in samples comprising of lipoproteins and free glycerol in the serum (p1 16).
In Mass fragmentation method, a reaction mixture is prepared by adding fix amount of glycerol trioleate and glycerol tripalmitate with the fix amount of serum sample [27-30]. Then the chloroform/methanol in the ratio of 2:1 v/v has been used for the extraction process in the above reaction mixture. The above mixture is subjected to thin layer chromatography and glycerol is obtained as resultant. This is followed by the acid hydrolysis in which the glycerol is splitted into trimethylsilyl derivative [31,32]. The concentration of unlabelled glycerol is determined using mass fragmentographer combined with smultiple ion detector analysers. The two peaks appeared at M-90 which corresponds to unlabelled trimethylsilyl and the peak at M-91 refers to labelled glycerol (p1 20).
Besides the above conventional methods there are some other methods including enzymatic determination of triglyceride with microtiter plates (p1 7), radio labelled glycerol tags were also used for determination of secretion of triglycerides in rats (p1 21). The merits and demerits associated with the above conventional methods are shown in the Table 1.
Biosensor
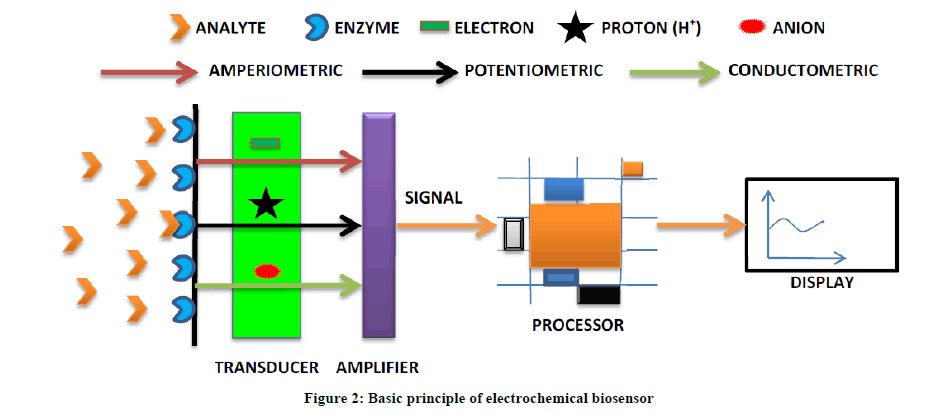
From the last 10 years biosensors have gained significant attention for their easy handling, less expensive, accurate and sensitive for real time monitoring of a variety of samples. Biosensor body consisted of basically three components. Biological material that can be immobilized for the detection of specific analyte, which can be an enzyme, whole cell, antibodies, cell receptors and many more. The second candidate of the biosensor body is the transducer [33-35]. A variety of transducers have been used for the fabrication of different types of biosensors according to the need of time [V 1]. Finally there is a display unit which provides the digital output of the present analyte concentration in the specific sample. The mode of detection biosensor of biosensor may be amperometric, potentiometric or conductometric as shown in the Figure 2.
Basic strategy behind TG biosensor
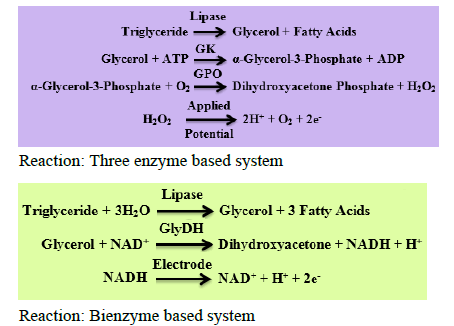
The electrochemical detection of TG can be done by analysing the two parameters. One is consumption of oxygen and the other is hydrogen peroxide (H2O2) production. In the early stages of the TG biosensor development a three enzyme system was used which includes Lipase/Glycerol Kinase (GK)/Glycerol-3-Phosphate Oxidase (GPO) [36-40]. As the three enzymes system adds to the cost of biosensor development the two enzyme system came in action. In this bienzyme system the enzymes Lipase and Glycerol Dehydrogenase are used in combination.
TG biosensor classification
Biosensor for triglyceride determination has been classified into membrane based, polymer based, field effect transistor based, modified screen printed electrode based and finally nanomaterial based as given in Figure 3.
Membrane based TG biosensors
Membranes are extensively used as a support for enzyme immobilization for detection of variety of analyte. Both the natural and artificial membranes are being exploited for the above purpose [41-45]. The basic theme behind this is confining enzyme behind semipermeable membrane which will allow passage of analyte through the press. The pore sized of membranes can also be adjusted at the time of synthesis. The membranes are biocompatible due to their selectivity and specificity. Different membrane supports have been used including egg shell membrane, Cellulose Acetate (CA), Polyvinyl Alcohol (PVA), Polyvinyl Chloride (PVC) etc. Membranes used for fabrication of TG biosensors include Egg shell membrane (p1 33), collagen membrane [28], PVC [31], CA [30], PVA [32] and AV membrane [29]. The artificial membranes are durable; flexible; possess selectivity for different biomolecules, functional at wide pH range resulting in enhanced sensitivity of the biosensors. The main drawback associated with the above membrane based TG biosensors is the blockage of the membrane pores by analyte [46-50]. This blockage is responsible for the hindrance in passage of analyte inside the membranes finally leading to the problem of membrane fouling (Table 2).
Polymer based TG biosensors
Polymers are also one of the suitable candidates for enzyme immobilization as variety has been observed in their physical and chemical properties. The polymer used in biosensor fabrication can be non-conducting or conducting polymer depending on the requirement and type of sensor to be developed. These supports have low cost, are biocompatible, provides a microenvironment to the immobilized enzyme and flexible [51,52]. Different chemical groups can be attached to the polymers using chemical treatment methods. This attachment of group of interest helps in stable immobilization and in other words enhances the stability of the biosensor. The non-conducting polymers such as Polyvinyl Alcohol (PVA), Mesocellular Silica Foam (MSF), polyamidoamine etc., suffers from the drawbacks as some times they act as barrier between electron transfer channel and finally hampers the electro catalytic activity of the biosensor. The use of conducting polymers solved the problem of hindrance in electron transport. The enzymes can be confined during process of electroplymerization. A uniform coating of polymer can be done on any kind of electrode surface. Conducting polymers include polyacrylamide, Polyethyleneimine (PEI), Polyaniline (PANI), Polypyrrole (PPy), chitosan etc. Poly (3,4-ethylenedioxythiophene) poly(styrene-sulfonate) (PEDOT:PSS) is one of the widely exploited conducting polymer in sensing technology as it has good immobilization capacity, highly stable, conductivity better than others and biocompatible for a variety of molecules (TG115 16). These conducting polymers have also been used in combination with a variety of nanoparticles in order to achieve improved conductivity of the biosensor. The PEDOT: PSS were used in combination with gold nanoparticles for successful determination of TG in serum (TG1) [53-56]. The conducting polymer supports used for fabrication of TG biosensors include: polyaniline used in combination with SWCNTs (p1 38), polypyrrole with gold [49] and conducting polymer hydrogel (p5) (Table 3).
Field Effect Transistor based (FET)
FETs have gained attention as the biosensors when the metal oxide body of the standard FETs have been modified by replacement of gate electrode with that of the reference electrode which is in contact with the gate oxide dipped in the solution [Bergveld p2] [34].
After this modification the enzyme layer can be easily immobilized near the gate oxide surface. The type of metal used and the pH of the solution influence the change in potential at the gate surface (Yuqing p2) [35]. It has been observed that the change of pH in the vicinity of gate is governed by the use of specific enzyme against their particular substrate depending upon the concentration of substrate present. As a result of this the ion selective FETs came in existence. The Enzyme based FETS (ENFETs) can determine the sensitivity, time of response and finally they can even be regenerated for further use (Jianrong p2) [36]. The enzyme immobilization in ENFETs can be achieved by crosslinking (Hermanson p2) [37], entrapment, and polymer layering of enzyme at the gate (LuO p2) [38]. The metal nanoparticles were used along with the immobilized enzyme in the fabrication of FETs based biosensors [Luo p2] [39]. The change in pH due to the presence of lipase enzyme was helped in the determination of presence of triglycerides using a microreactor ISFET (ion selective) biosensor reported earlier (Pijanewska p2) [40]. The magnetic nanoparticles had also been exploited successfully with immobilized lipase enzyme for the detection of triglycerides. Then these magnetic nanoparticles were attached to the surface gate of ISFET. A covalent immobilization of lipase was done with the NiFe2O4 magnetic nanoparticles. This biosensor detected the change in pH which is generated when lipase came in close proximity of triglycerides. The biosensor showed a linearity of 5-30 mM, response time was less than 5 minutes and a good reusability, pH-FET [52], Mesoporous Silica [55], Porous Silica [53,54], and Silicon Nitride/EISCP (Table 4) [56].
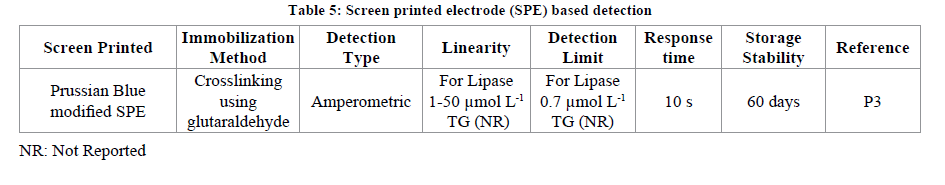
Screen Printed Electrode (SPE) based detection
SPE are the electrodes which are compact and have the three electrodes i.e., working, counter and the reference on the same screen. The biological material (enzyme, cell, receptor etc.) can be immobilized on the working electrode spot of the screen printed electrode. The SPE have been used in fabrication of variety of biosensors (v2). The application of SPE for TGs determination has also been reported. A Prussian blue (PB) modified SPE has been fabricated for the determination of TG in olive oil or sunflower oil real samples. For the fabrication of the above said SPE electrode first of all the working spot of the electrode has been modified with PB followed by immobilization of mixture of NADH oxidase and glycerol dehydrogenase using glutaraldehyde as cross linking agent. The biosensor showed a good linearity from 1-50 μmol L-1 with a minimum detection limit of 0.7 μmol L-1. When the developed biosensor was exploited against the sonicated sample of olive oil or vegetable oil a percent recovery of 99% and with that of non-sonicated samples the recovery was 81% was observed (p3) (Table 5).
Nanostructured material based TG biosensors
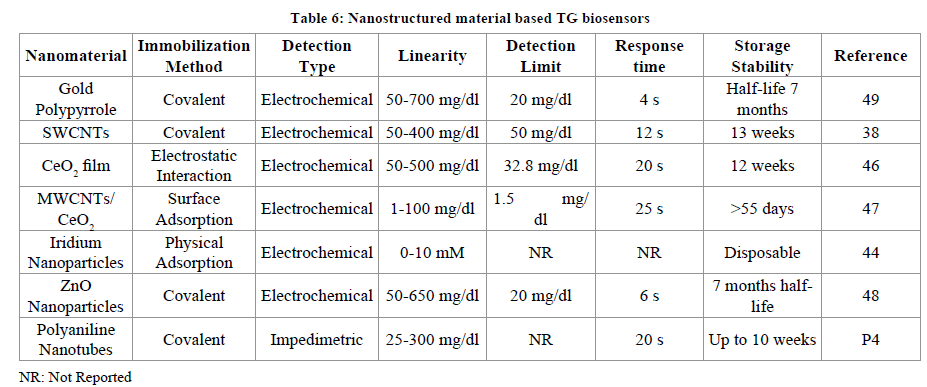
All the above supports used for biosensor fabrication suffer from one or the other drawbacks related to sensitivity, accuracy, false signals, interference due to metal ions and other electroactive species. Due to above limitations nanomaterial attracted researchers for using nanomaterial based working electrodes in detection of TGs. These nanostructured materials have been categorised into zero dimensional (nanoparticles, quantum DotS), one dimensional (carbon nanotubes, nanowires) and two dimensional (graphene sheets, platelets) [V nano].
Among these the metallic nanoparticles have been much explored. The nanoparticles possess high thermal stability, non-toxic; can be easily synthesized in laboratory, biocompatible with variety of molecules, works better even at low temperature, highly electrocatalytic, and high surface area. Zinc Oxide (ZnO), titanium oxide (TiO2), cerium oxide (CeO2), tin oxide (SnO2), zirconium oxide (ZrO2) etc. are some of them. Sol gel based metallic nanoparticles SnO2 [19], TiO2 [20,21], ZnO [17,18] have also been used in fabrication of biosensors. The CeO2 nanoparticles possess a wide band gap of 3.4 eV, better mechanical strength, conductive towards oxygen ions, isoelectric point (9.2). Nanomaterial based TG biosensors include Gold nanomaterial/polypyrrole [49], Single walled carbon nanotubes in combination with conducting polymer polyaniline [38], sol gel derived CeO2 coated as film on glass plate layered with indium-tin-oxide (ITO) [46], nanocomposite of MWCNTs/CeO2 nanoparticles [47], Iridium nanoparticles [44], Zinc oxide nanoparticles [48] and polyaniline nanotubes deposited onto ITO coated glass plate (Table 6) [43].
Conclusion
Triglycerides being responsible for serious diseases such as artery blockage, heart attack, atherosclerosis etc. It is very necessary to monitor elevation in blood stream for early diagnosis and prevention from linked disorders. The available conventional methods are not fast, accurate, other metabolites may affect determination, sample preparation required and last but not the least requires expensive equipments with trained personals. On the other hand biosensors have been fabricated which overcome the limitations of conventional analytical methods. Membranes, conducting polymers, Ion Selective FETs, SPEs and nanomaterials have been used for TG determination. But these also suffer from one or the other limitation related to membrane fouling, less sensitive, narrow linearity range, reduced storage stability etc. Nanomaterials have been used such as nanoparticles, single walled carbon nanotubes, polyaniline nanotubes, multi walled carbon nanotubes etc. with new and promising horizon in fabrication strategy of biosensors. High electrocatalytic properties, large surface area, requirement of low potential for detection have accelerated TG biosensor generation. The merits and demerits of different supports used for immobilization of TG have also been discussed in table. The nanomaterials such as graphene nanosheets, Nanorods, nanowires, platelets must also be exploited for their use as supports for development of TG biosensors and overcome the demerits.
References
[1] B.G. Nordestgaard, M. Benn, P. Schnohr, A. Tybjaerg-Hansen, J. Am. Med. Assoc., 2007, 298-299.
[2] J.S. Cohn, C. Marcoux, J. Davignon, Arterioscler. Thromb. Vasc. Biol., 1999, 19, 2474.
[3] P. Fossati, L. Prencipe, Clin. Chem., 1982, 28, 2077-2080.
[4] M. Okazaki, N. Komoriya, H. Tomoike, N. Inowe, S. Itoh, S. Hoosaki, J. Chromatogr. B. Biomed. Sci. Appl., 1998, 709, 179-187.
[5] http://www.heart.org/HEARTORG/
[6] G. Kessler, H. Lederer, L.T. Skeggs Jr. (Ed.), Mediad, NY, USA, 1966, 341-344.
[7] M. Werner, D.G. Gabrielson, E. John, Clin. Chem., 1981, 22, 268-271.
[8] V.K.S. Shukla, Prog. Lipids. Res., 1988, 27, 5-38.
[9] S.G. Klotzsch, J.R. McNamara, Clin. Chem., 1990, 36, 1605-1613.
[10] J.W. Brunner Kreeft, B. Leijnse, J. Clin. Chem. Biochem., 1986, 24, 445-459.
[11] V. Handel, D.B. Zilversmit, J. Lab. Clin. Med., 1957, 50, 152-157.
[12] R. Asmis, E. Biihler, J. Jelk, G.K. Fred, J. Chromatogr. B: Biomed. Sci. Appl., 1997, 691, 59-66.
[13] I. Bjorkhem, K. Sandelin, T. Anders, Clin. Chem., 1982, 28, 1742-1744.
[14] R.B. Shireman, J. Durieux, Lipids., 1993, 28, 151-155.
[15] H.C. Lai, J.B. Lasekan, H. Yang, M.K. Clayton, D.M. Ney, Lipids., 1991, 26, 824-830.
[16] V. Dhull, A. Gahlaut, N. Dilbaghi, V. Hooda, Biochem. Res. Int., 2013, 18.
[17] J. Narang Minakshi, M. Bhambi, C.S. Pundir, Int. J. Biol. Macromol., 2010, 47, 691-695.
[18] H. Winartasaputra, S.S. Kutan, G.C. Cuilbault, Anal. Chim., 1982, 54, 1987-1990.
[19] J. Narang, M. Bhambi, Minakshi, C.S. Pundir, Anal. Lett., 2010, 40, 1-10.
[20] C.S. Minakshi, Pundir, Sens. Actuators B: Chem., 2008, 133, 251-255.
[21] C.S. Pundir, S. Singh, Bharvi, J. Narang, Clin. Biochem., 2010, 43, 467-472.
[22] D. Compagnone, M. Esti, M.C. Messia, E. Peluso, G. Palleschi, Biosens. Bioelectron., 1998, 13, 875-880.
[23] B. Adhikari, S. Majumdar, Progress in Polymer Science (Oxford), 2004, 29, 7, 699-766.
[24] T. Kaku, H.I. Karan, Y. Okamoto, “Anal. Chem., 1994, 66, 8, 1231-1235.
[25] L. Coche-Guerente, S. Cosnier, C. Innocent, P. Mailley, Analytica. Chimica. Acta., 1995, 311, 1, 23-30.
[26] J. Liu, M. Agarwal, K. Varahramyan, 2008, 135, 195-199.
[27] S.M. Richardson-Burns, J.L. Hendricks, B. Foster, L.K. Povlich, K. Dong-Hwan, D.C. Martin, Biomaterials., 2007, 28, 1539-1552.
[28] A. Phongphut, C. Sriprachuabwong, A. Wisitsoraat, A. Tuantranont, S. Prichanont, P. Sritongkham, Sensors and Actuators B: Chemical, 2013, 178, 501-507.
[29] C. Dhand, P.R. Solanki, M. Datta, B.D. Malhotra, Electroanalysis., 2010, 22, 2683-2693.
[30] J. Narang, N. Chauhan, P. Rani, C.S. Pundir, Bioprocess. Biosyst. Eng., 2013, 36, 425-432.
[31] L. Li, Yaqun Wang, L. Wang, Y. Shi, W. Cheng, Y. Shi, G. Yu, Nano Letters., 2015, 15, 1146-1151.
[32] P. Bergveld, Sens. Actuator., 2003, 88, 1-20.
[33] M. Yuqing, G. Jianguo, C. Jianrong, Biotechnol. Adv., 2003, 21, 527-534.
[34] C. Jianrong, M. Yuqing, H. Nongyue, W. Xiaohua, L. Sijiao, Biotechnol. Adv., 2004, 22, 505-518.
[35] G.T. Hermanson, Bioconjugate Techniques, 1996, Academic Press, San Diego.
[36] X.L. Luo, J.J. Xu, W. Zhao, H.Y. Chen, Sens. Actuator., 2004, B 97, 249-255.
[37] Y.C. Luo, J.S. Do, Biosens. Bioelectron., 2004, 20, 15-23.
[38] D.G.P. Pijanewska, A. Baraniecka, R. Wiater, G. Ginalska, J. Labareswski, W. Torbicz, Sens. Actuator. B., 2001, 78, 263-266.
[39] A. Vijayalakshmi, Y. Tarunashree, B. Baruwati, S.V. Manorama, B.L. Narayana, R.E.C. Johnson, N.M. Rao, Biosens. Bioelectron., 2008, 23, 1708-1714.
[40] M. Nakako, Y. Hanazato, M. Maeda, S. Shiono, Anal. Chim. Acta., 1986, 185, 179-185.
[41] S. Setzu, S. Salis, V. Demontis, A. Salis, M. Monduzzi, G. Mula, Phys. Stat. Sol., 2007, 204, 1434-1438.
[42] R. Reddy, R.C. Anju, B. Enakshi, Biosens. Bioelectrons., 2001, 16, 313-317.
[43] I. Basu, R.V. Subramanian, A. Mathewa, A.M. Kayasthac, A. Chadha, E. Bhattacharya, Sens. Actuators., 2005, 107, 418-423.
[44] D.G. Pijanewska, A. Baraniecka, R. Wiater, G. Ginalska, J. Lobarzewski, W. Torbicz, Sens. Actuators., 2001, 78, 263-266.
[45] V. Dhull, N. Dilbaghi, V. Hooda, Int. J. Pharm. Pharmaceut. Sci., 2015, 7, 17-24.
[46] I.B. Rejeb, F. Arduini, A. Amine, M. Gargouri, G. Palleschi, Analytica. Chimica. Acta., 594, 2007, 1-8.
[47] N. Jia, Q. Zhou, L. Liu, M.M. Yan, Z.Y. Jiang, J. Elect. Chem., 2005, 580, 213-221.
[48] J. Yu, H. Ju, Anal. Chem., 2002, 74, 3579-3583.
[49] Q. Li, G. Luo, J. Feng, Q. Zhou, L. Zhang, Y. Zhu, Electroanalysis., 13, 2001, 413-416
[50] J.X. Wang, X.W. Sun, A. Wei, Y. Lei, X.P. Cai, C.M. Li, Z.L. Dong, Appl. Phys. Lett., 2006, 88, 233106-233109.
[51] A. Wei, X.W. Sun, J.X. Wang, Y. Lei, X.P. Cai, C.M. Li, Z.L. Dong, W. Huang, Appl. Phys. Lett., 2006, 89, 123902-123905.
[52] P.R. Solanki, C. Dhand, A. Kaushik, A.A. Ansari, K.N. Sood, B.D. Malhotra, Sens. Actuators., 2009, 141, 551-556.
[53] M.R. Ganjali, F. Faridbod, E. Nasli-Esfahani, B. Larijani, H. Rashedi, P. Norouzil, Int. J. Electrochem. Sci., 2010, 5, 1422–1433.
[54] W.Y. Liao, C.C. Liu, T.C. Chou, Analyst., 2008, 133, 1757-1763.
[55] J. Narang, C.S. Pundir, Int. J. Biol. Macromol., 2011, 49, 707-715.
[56] C. Dhand, P.R. Solanki, K.N. Sood, M. Datta, B.D. Malhotra, Electrochem. Commun., 2009, 11, 1482-1486.



