Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 2
A Novel Route to the Synthesis of Acridine Derivatives and Assay of their Antiviral Activity
Soniya Singh*, Yamini Pandey and V. K. PandeySoniya Singh, Department of chemistry, University of Lucknow, Lucknow-226007, India, Email: soniyasingh.chem@gmail.com
Received: 13-Sep-2020 Accepted Date: Feb 19, 2021 ; Published: 26-Feb-2021
Abstract
A simple and convenient procedure was adopted for the synthesis of N-(3-acridin-9-yl-4-chloro/hydroxy-phenyl-alkyl)-arylamides/imides starting from 5-(arylamido/imido-alkyl)-2-chloro/2-hydroxy benzoic acids. The compounds were obtained in the yields ranging from 40 to 45% and exhibited less pronounced antiviral activity.
Keywords
Arylamido/imidoalcohols, 2 substituted anthranilic acids, Antiviral activity
Introduction
Acridine derivatives as antiviral agents have been less investigated however their antibacterial activity has been fully established [1-3]. The acridine compounds exert both bactericidal and bacteriostatic actions against both gram positive and gram Negative organisms and are not inhibited by the serum [4].
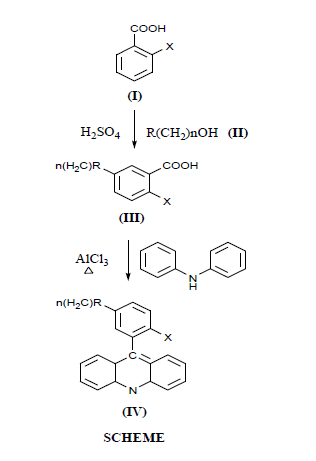
It has been observed that bacteria can acquire resistance to acridines on prolonged use [5,6]. In addition, later studies proved the usefulness of acridines against malarial infections. Some such acridines are chemically effective and are available in the market [7-9]. Our ongoing programme in the design and development of the potential bioactive compounds led us to undertake the synthesis of some acridine derivatives for studying their antiviral activity (Scheme).
Experimental
Arylamido/imidoalcohols (ll)
Literature methods [10-12] were followed for the synthesis of arylamido/imidoalcohols.
5-(Arylamido/imidomethyl) -2 chloro/ 2 hydroxy benzoic acid (lll)
A mixture of an arylamido/imidoalcohols (ll) (0.01 Mole) and orthosubstituted benzoic acid (0.01 mole) was resolved in conc H2SO4 (50 ml) by stirring. During the course of dissolution the contents were cooled. The solution thus obtained was stirred mechanically for one hour and left under refrigeration overnight. It was poured into Ice cold water 250 ml slowly and carefully after each addition the stiring was done. When the addition was completed, the separated solid mass was allowed to settle down. It required about half an hour. The solid was filtered off and was successively with cold water and dried in vacuo. The crude amido/imidoalkylated acid thus obtained was recrystallized from acetone 5 (Benzoylamino-methyl) 2-chlorobenzoic acids, m.p. 134°C (133 to 134°C) [13] yield 75%
5-(1,3Dioxo- 1,3 dihydro-Isoindol-2-yl-methyl) 2- hydroxy-benzoic acid m.p. 167-168oC [169°C] [14] ,yield 70%.
5-(Benzoylamino-methyl)-2-hydroxy benzoic acid, m.p. 109-110°C [110°C] [15] yield 77%.
N-(3-Acridin-9-yl-4-chloro/4-hydroxy-phenyl-alkyl)-arylamides/imides (IV)
A mixture consisting of properly powdered diphenyl amine(0.9mole) and 5-(arylamido/imido-alkyl) -2- chloro/ 2-hydroxy benzoic acid (III) (0.0l mole) and anhydrous aluminium chloride (1.0 g) was heated in such a manner that the temperature did not increase 160o C for a longer period of times (11 hrs). Subsequently, dark green mixture was cooled till it attained the room temperature. The resulting solid was thoroughly washed initially with sodium bicarbonate (10%) solution to dissolve any unreacted carboxylic acid. It was further washed repeatedly with water and dried in vacuo. The crude acridine derivatives thus synthesized, were recrystallized from benzene and are recorded in Table 1(Because of the labrymatric nature of the acridine derivatives, these compounds were handled with much care.)
| Compd. No | R | X | m.p. 0C |
Yield | Colour | Molecular Formula | Molecular Weight | Analysis Nitrogen% | |
|---|---|---|---|---|---|---|---|---|---|
| Calcd. | Found | ||||||||
| 1. | benzamido | Cl | 110-111 | 45 | Dark brown | C27H19N2OCl | 422.5 | 6.63 | 6.28 |
| 2. | benzamido | OH | 125 | 40 | Dark brown | C27H20N2O2 | 404 | 6.93 | 6.56 |
| 3. | phthalimido | OH | 112 | 40 | Dark brown | C28H18N2O3 | 430 | 6.51 | 6.44 |
Table 1: Characterization data of N-(3-acridin-9-yl-4-chloro/4-hydroxy-phenyl-methyl)-arylamides/imides (IV).
IR(KBr, cm-1) : 1690 ( sec. amide C= O), 1635(C=N), 3505(ArOH), 3420 (amide NH)
1HNMR (DMSO, d6) (δppm) : 6.75-7.82 (m, 16H,ArH), 3.85(s, 2H,C-CH2-NH), 8.85(brs,1H,CONH), 5.50 (s,1H,ArOH)
13CNMR (DMSO,d6) (δppm): 43.4,115.2, 118.5, 121.4, 125.2, 127.3, 128.6, 130.5, 131.4, 135.6, 147.7, 165.7, 170.5
MS (FAB) : 404(M+), other Important peaks appeared at m/z 404, 587, 226, 178, 134, 127, 120, 107, 105(base peak), 101.
Antiviral activity
All the three target compounds were evaluated for their antiviral activity against Japanese encephalites virus (JEV), a RNA virus of greater pathogenicity obtained from National Institute of virology, Pune (India) in vitro [16,17]. These compounds were also screened for their antiviral activity against the plant virus viz; Tobacco mosaic virus (TMV). From the Nicotiana Glutinosa plant both in vitro and in vivo [18,19]. The antiviral activity data of these compounds are recorded in the Table 2 and Table 3.
| Compound number | R | X | Dose (µg/ml) |
CT50 (µg/ml) |
EC50 (µg/ml) |
TI | %CPE inhibition |
|---|---|---|---|---|---|---|---|
| 1 | benzamido | Cl | 500-4 | 500 | - | - | - |
| 2 | benzamido | OH | 500-4 | 500 | - | - | - |
| 3 | phthalamido | OH | 500-4 | 500 | 250 | 02 | 20 |
CT50=50% cytotoxic concentration, EC50=50% effective concentration,
TI= Therapeutic index, CPE= Cytopathic effect
Table 2: Anti-Japanese encephalitis virus (JEV) activity data of N-(3-acridin-9-yl-4-chloro/4-hydroxy-phenyl-methyl)-arylamides/imides
| Compound no. | R | X | Percent inhibition of TMV | |
|---|---|---|---|---|
| Invitro | Invivo | |||
| 1 | Benzamido | Cl | 30 | 10 |
| 2 | Benzamido | OH | 20 | - |
| 3 | Phthalimido | OH | 50 | 30 |
Table 3: Anti-Tobacco mosaic virus (TMV) activity data of N-(3-acridin-9-yl-4-chloro/4-hydroxy-phenyl-methyl)-arylamides/imides
Results and Discussions
Anti JEV activity data incorporated in Table-II clearly suggest that these compounds having substituents in the phenyl group joined at 9 position of the acridine nucleus are unable to provoke good activity. Only a very lower order of anti JEV activity in vitro was demonstrated by the compound no. 3. However some correlation can be made with regard to anti-viral activity and the molecular structure of the compound. Thus the compound no.2 bearing R= benzamide and X'= OH substituents was found completely devoid of any measurable degree of anti JEV activity while compound no. 3 having R= phthalimido and X=OH showed observable magnitude of anti JEV activity (20% net protection). This observation clearly suggests that a phthalimido substituent is comparatively more appropriate than a benzamido substituent. It is suggested that these compounds are unable to penetrate the glycoprotein of the JEV to exert their desirable biologic effect and are unable to find the better fit at the receptor sites.
All the three acridine compounds displayed moderate to lower order of antiviral activity against TMV in vitro. However, in vivo antiviral activity against TMV was found to be less pronounced. From the anti -TMV activity data recorded in Table 3, it is clear that only one compound out of three , is showing some satisfactory virus inhibitory properly both in vitro and in vivo. Thus, the compound number 3 bearing R= phthalimido and X=OH exhibited antiviral activity to the extent of 50% in vitro and 30% in vivo. The acridine compound no. 2 having R= benzamido and X=OH showed a comparatively low order of activity. This compound provoked only 20% inhibition of TMV in vitro and no measurable degree of anti TMV activity in vivo could be detected. However the compound number one containing R=benzamido and X=Cl showed 30% and 10% anti TMV activity both in vitro and in Vivo respectively. It seems quite reasonable to suggest here that such compounds with other substituents need further probe for generating potential candidate molecules.
Acknowledgement
The authors are grateful to the Head, Chemistry Department Lucknow University, Lucknow for providing necessary laboratory facilities and to the Director, Central Drug Research Institute (CDRI), Lucknow for providing elemental, spectral and biological activity data.
References
- CH Browning and W Gilmour. J Patholi Bacterial. 1913,18:144.
- A Albert. The Acridines, Their preparations, Properties and uses, Arnold London. 1951.
- AAlbert. Selective Toxicity, Ed. 3, Wiley, New York, 1965, p. 192-205.
- ]G Sykes., Disinfection and Sterilization, Ed. 2, Lippincott, Philadelphia, 1965, p. 357.
- MS Thornley and J Yudkin, J. Gen. Microbial, 1959, 20: p. 355, 356.
- J Sinai and J Yudkin. J Gen Microdial. 1959, 20: p. 373
- BN Robinstien, Arch. Schiffs Trop Hyg. 1936, 40: p. 167.
- NN Dykhanov, GA Gorlach and VP Sergovaskaya. Med Prom SSR. 1960, 14: 22.
- DM Besly and AA Goldburg, J Chem Soc. 1954, 2448.
- SR Buc. J Amer Chem Soc. 1947, 69:p. 254-256.
- EJ Sakellaries. Amer Chem Soc. 1948, 70: p. 2822.
- M Sekiya and K Yto. Chem Pharm Bull (Tokyo). 1963,11:p. 888-891.
- A Shukla. VK Pandey, G Saxena et al., Acta Pharm. 1995, 45: p. 29-35.
- VK Pandey, KC Khulde, G Sacena et al., Indian Drugs. 1993, 30: p. 513-514.
- VK Pandey, HC Lohani and AK Agarwal, India Drugs, 1984, 21: p. 135-148.
- LJ Reedand H Muench. Amer J Hyg. 1938, 77: p. 493-497.
- RA Sidwell and TN Huffmann. Appl Microbial. 1971, 22:p. 791-801.
- HN Verma, LP Awasthi, SP Singh. 1979, 6: p. 86.
- VK Pandey and J Kumar. Ind J Act Chem. 2006,16: p. 65-66.