Mini Review - Der Pharma Chemica ( 2021) Volume 13, Issue 9
A Molecular Docking Study Bring Golden Era in Pharmaceutical Drug Design and Discovery
Ashish Pandey*, Achal Mishra and Davendra KumarAshish Pandey, Faculty of Pharmaceutical Science, Shri Shankaracharya group of Institute, Bhilai (C.G.), India, Email: ashispharma@gmail.com
Received: 26-Jul-2021 Accepted Date: Sep 24, 2021 ; Published: 30-Sep-2021
Abstract
Molecular docking has become an increasingly important tool for drug discovery. In this review, we present a brief introduction of the available molecular docking methods, and their development and applications in drug discovery. The relevant basic theories, including sampling algorithms and scoring functions, are summarized. The differences in and performance of available docking software are also discussed.
Keywords
Molecular Modelling Molecular docking, AutoDock, Molegro Virtual Docker, Surflex-Dock, 3D-Dock, The Gemdock
Introduction
The development of new drug Pharmaceutical industry taking long year. In long year ago drug discovery thought to be a viable solution and then at least after 15 year clinical trial to came into market. It means pharmaceutical industry facing various challenges during the development of new molecule1. But now a day we understand the physiology of drug and develop the molecule to cure them. The basic psiosiology of drug/ ligand that are small molecular structure that bind with large macromolecule (Receptor /Protein) form a complex and produce pharmacological effect.
Forthcoming of high performance computing and computational biology give new turn and achieve the goal of drug discovery.1In structure-based design the structures of known target proteins are used to discover new compounds of therapeutically relevance. The approaches can be classified roughly into two categories de novo design and docking. It has emerged as a popular methodology for drug design it combine with computational chemistry and computer graphics. The former method designs new ligands to fit the protein target, whereas the latter is used to decide whether existing compounds possess good steric and chemical complementarities to the given protein [1].
Today, the field of drug development may seem more fertile than ever before, with vast amounts of information from genomic and proteomic studies facilitating the finding of new targets, the usage of rational combinatorial chemistry for the production of libraries of compounds, the generation of genetically modified animal models for the development and testing of new drugs, and the possibility of using ultra-high-throughput test techniques for the screening of large libraries. However, despite all these advances, the revolutionary era of drug design has not arrived yet [2-4]. There is no unique solution to a drug design problem. The appropriate experimental techniques or computational methods to use will depend on the characteristics of the system itself and the information available [5-8].
Drug target are Enzyme and Receptor. Drugs combine with enzyme and receptor form stable complex and produce pharmacological effect. Ligand is a small molecule, which interacts with protein's binding sites. Binding sites are areas of protein known to be active in forming of compounds. There are several possible mutual conformations in which binding may occur. These are commonly called binding modes [9].
Molecular docking is an invaluable tool in modern drug discovery. This method is mainly guided by molecular recognition and by the forces between organic molecules (drugs) and the protein (receptor). The associations between biologically relevant molecules such as proteins, nucleic acids, carbohydrates, and lipids play a central role in signal transduction. Furthermore, the relative orientation of the two interacting partners may affect the type of signal produced (e.g., agonism vs antagonism). Therefore docking is useful for predicting both the strength and type of signal produced. Docking is frequently used to predict the binding orientation of small molecule drug candidates to their protein targets in order to in turn predict the affinity and activity of the small molecule. Hence docking plays an important role in the rational design of drugs. Given the biological and pharmaceutical significance of molecular docking, considerable efforts have been directed towards improving the methods used to predict docking [10-12].
Molecular Docking
Docking techniques, designed to find the correct conformation of a ligand and its receptor, have now been used for decades [11–16]. The process of binding a small molecule to its protein target is not simple; several entropic and enthalpic factors influence the interactions between them. The mobility of both ligand and receptor, the effect of the protein environment on the charge distribution over the ligand, [17] and their interactions with the surrounding water molecules, further complicate the quantitative description of the process. The idea behind this technique is to generate a comprehensive set of conformations of the receptor complex, and then to rank them according to their stability. The most popular docking programs include DOCK [18,19] AutoDock [20] FlexX [21] GOLD [22] and GLIDE [23,24] among others.
Types of Docking- Protein-Protein docking and Ligand-Protein docking protein–protein interactions, which is very significant for understanding biochemical processes, e.g. signal transduction, cell regulation and immune response [25].
Two approaches are particularly popular within the molecular docking community. One approach uses a matching technique that describes the protein and the ligand as complementary surfaces [21,22]. The second approach simulates the actual docking process in which the ligand-protein pair wise interaction energies are calculated [26].
Software used for molecular docking
AutoDock
The program AutoDock was developed to provide a procedure for predicting the interaction of small molecules with macromolecular targets. The motivation for this work arises from problems in the design of bioactive compounds, and in particular the field of computer aided drug design. Progress in biomolecular x-ray crystallography has provided a number of important protein and nucleic acid structures that could be targets for bioactive agents in the control of disease, or as agricultural agents. The precise interaction of such agents or candidates is important in the development process. Our goal has been to provide a computational tool to aid in this process [27].
The procedure developed for AutoDock uses a Monte Carlo simulated annealing technique for configurational exploration with a rapid energy evaluation using grid based molecular affinity potentials, thus combining the advantages of a large search space and a robust energy evaluation. This has proven to be a powerful approach to the problem of docking a flexible substrate into the binding site of a static protein [28].
Molegro Virtual Docker
Molegro Virtual Docker is an integrated platform for predicting protein – ligand interactions. It handles all aspects of the process, from preparing the molecules to determining the potential binding site of the target protein, and predicting the binding mode of the ligand. Molegro Virtual Docker offers high-quality docking based on novel optimization techniques combined with a user interface experience focusing on usability and productivity [29].
Surflex-Dock
Surflex-Dock offers unparalleled enrichments in virtual high-throughput screening1 combined with state-of-the-art speed, accuracy and usability. It uses an empirical scoring function (based on the Hammerhead docking system) that has been updated and re-parameterized with additional negative training data, along with a search engine that relies on a surfacebased molecular similarity method [30-34].
3D-Dock
3D-Dock is a suite of programs designed to enable computational prediction of protein- protein docking. It does this in several steps.
The Gemdock
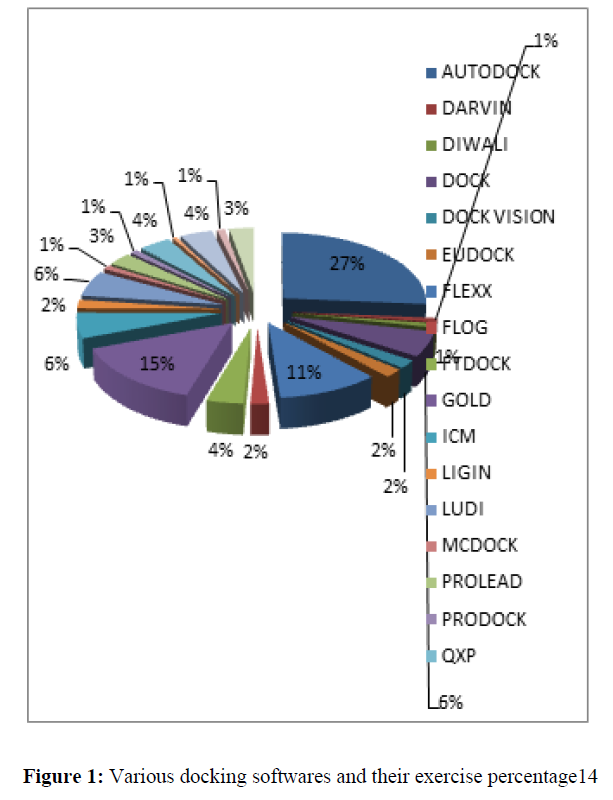
The Gemdock software is available on the Web at http://gemdock.life.nctu.edu.tw. This program uses an empirical scoring function and an evolutionary approach that is more robust than standard evolutionary approaches [25–27] with regard to several specific domains [28–31]. The GEMDOCK energy function consists of electrostatic, steric and hydrogenbonding potentials. Steric and hydrogen bonding potentials use a linear model that is simple and recognizes potential complexes rapidly. The core idea of this evolutionary approach is to design multiple operators that cooperate using a family competition paradigm that is similar to a local search procedure [32-34] (Figure 1).
An algorithm (derived from the name of mathematician al-Khwārizmī) is an effective method for solving a problem expressed as a finite sequence of steps.
Yucca
A new rigid docking algorithum. Its scoring function called piecewise linear potential [13,14]. In the PLP model [16,17] atoms are classified into the following four types: H-bond donor, H-bond acceptor, H-bond donor/acceptor, and nonpolar. Each pair of interacting atoms is then assigned one of the three interaction types: a H-bond interaction between donors and acceptors, a repulsive interaction for donor ± donor and acceptor ± acceptor contacts, and a dispersion for other contacts. The energy of each interaction type is represented by a piecewise linear function.
References
- Grosdidier A, Zoete V and Michielin O. Proteins. 2007, 1: p. 67.
- Brown D and Superti-Furga G. Drug Discov Today. 2003, 8: p. 1067.
- Drews J. Drug Discov Today, 2003. 8: p. 411.
- Lahana R. Drug Discov Today. 1999, 4: p. 447.
- Jain AN. Curr Opin Drug Discov Devel. 2004, 7: p. 396–403.
- Oprea TI. Curr Opin Chem Biol. 2004, 8: p. 349–358.
- Shoichet BK. Nature. 2004, 432: p. 862–865.
- Stahura FL and Bajorath J. Curr Pharm Des. 2005, 11: p. 1189–1202.
- Aatu K and Janne O. Protein docking. 2002, p. 3-17
- Kellenberger E, Rodrigo J, Muller P et al., Proteins. 2004, 57: p. 225-242.
- Welch W, Ruppert J and Jain AN. Chem Biol. 1996, 3: p. 449-62.
- United States Patent No. 6,470,305; 20.
- Jain AN. J Comput Aided Mol Des. 2000, 14: p. 199–213
- Pham TA and Jain AN. J Med Chem. 2005.
- Alonso A, Andrey Bliznyuk and Jill Gready E. Medicinal Research Reviews. 2006, 26.
- Brooijmans N and Kuntz ID. Annu Rev Biophys Biomol Struct. 2003, 32: p. 335–373.
- Cummings MD, Des Jarlais RL, Gibbs AC et al., J Med Chem. 2005, 48: p. 962–976.
- Halperin I and Nussinov R. Proteins. 2002, 47: p. 409–443.
- Kitchen DB, Decornez H, Furr JR et al., Nat Rev Drug Discov. 2004, 3: p. 935–949.
- Shoichet BK, McGovern SL, Wei B et al., Curr Opin Chem Biol. 2002, 6: p. 439–446.
- Docking and md simulations in drug design, 561.
- Taylor RD, Jewsbury PJ and Essex EW. J Comput Aided Mol Des. 2002, 16: p. 151–166.
- Cho AE, Guallar V, Berne BJ et al., J Comput Chem. 2005, 26: p. 915–931.
- Li CH, Ma HX, Chen WZ et al., Protein Engineering, 2003, 16: p. 265–269.
- Meng EC, Shoichet BK and Kuntz ID. J Comp Chem. 2004, 13: p. 505–524.
- Morris GM, Goodsell DS, Halliday RS et al., J Comp Chem. 1998, 19: p. 1639–1662.
- Feig M, Onufriev A and Lee MS,et al., J Comp Chem. 2004, 25: p. 265–84.
- Kellenberger E, Rodrigo J, Muller P et al., Proteins. 2004, 57: p. 225-242
- Welch W. Ruppert J and Jain AN. Chem Biol. 1996, 3: p. 449-62.
- United States Patent No. 6,470,305; 20.
- Jain AN. J Comput Aided Mol Des. 2000, 14: p. 199–213
- Pham TA and Jain AN. J Med Chem. 2005.
- Gehlhaar DK, Verkhivker GM, Rejto PA et al., Chem Biol. 1995, 2: p. 317.
- Verkhivker GM, Bouzida D, Gehlhaar DK et al., J Comput Aided Mol Des. 2000, 14: p. 731.