Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 4
A Highly Efficient Method for Synthesis of Novel 6,6'-(1,4-Phenylene)bis(4-phenylpyrimidin-2-OL/-2-amine/2-thiol and Evaluation of their Biological Activities
Shobha Bansal and Prabal P Singh*
Department of Chemistry, GLA University, 17 km stone NH-2 Delhi-Mathura Highway Chaumuhan Mathura-281406, UP, India
- *Corresponding Author:
- Prabal P Singh
Department of Chemistry
GLA University
17 km stone NH-2 Delhi-Mathura Highway Chaumuhan Mathura-281406, UP, India
Abstract
Three novel bis-heterocycles namely 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-ol), 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-amine) and (6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-thiol) have been synthesized by treating chalcone and bisnucleophiles over MgFe2O4 magnetic nanoparticle to yield the bis-pyrimidine analogues in good yields in very short reaction time. The newly synthesized compounds were exhibited a good range of antimicrobial activities.
Keywords
Antimicrobial activities, Heterogeneous catalyst, Magnetic nanoparticles, Bis-heterocycles
Introduction
Structural sub units of five and six membered heterocycles that are abundant in nature exist in many natural products like vitamins, hormones, amino acids, alkaloids, hemoglobin and antibiotics [1]. Nucleic acid hydrolysis produces several pyrimidines like uracil, thymine and cytosine bases. Pyrimidine derivatives have shown anticancer [2], antimicrobial [3], anticonvulsant [4], antiviral [5], antineoplastic [6], antihistamic [7], antidiabetic [8], antiallergic [9], antipyretic [10] and antihypertensive [11] activities. The development of drug resistance and appearance of undesirable side effect of certain antibiotics has forced the researcher to look for new antimicrobial agents, with a goal to discover new chemical structural frameworks which may overcome the above mentioned shortcomings.
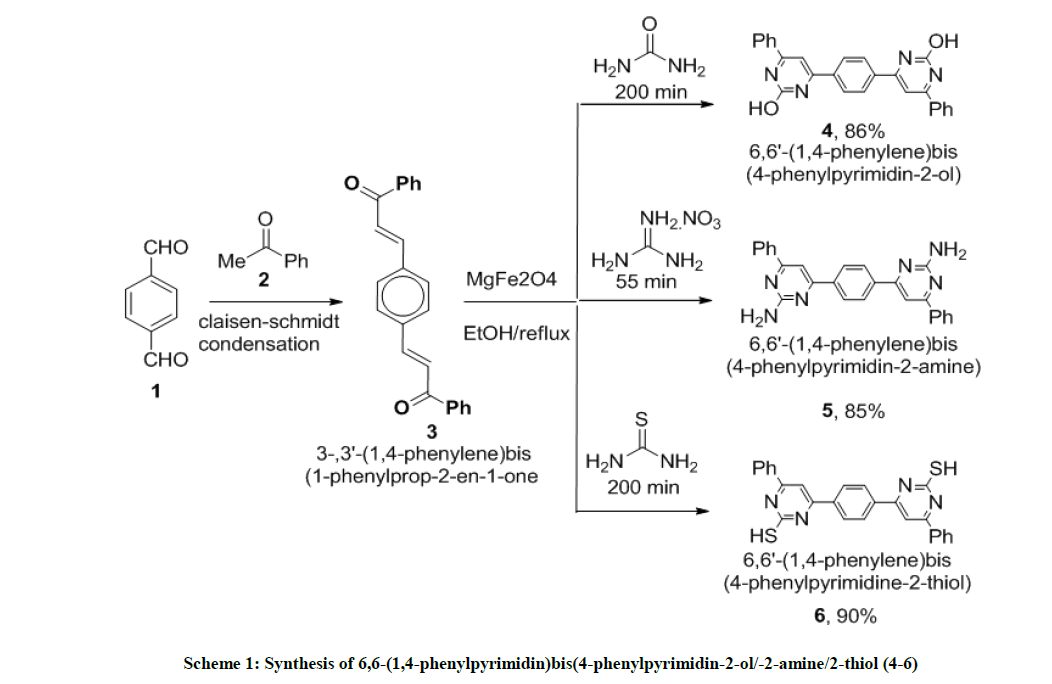
In present study we report synthesis of three novel bis-pyrimidines analogues like 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-ol), 6,6'-(1,4- phenylene)bis(4-phenylpyrimidin-2-amine) and (6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-thiol) efficiently synthesized by green approach using cheaper, reusable, non-toxic magnetically separable MgFe2O4 as nano catalyst with reduced reaction time. The potency of these newly synthesized compounds were examined against both for Gram-positive and Gram-negative strain bacteria along with their antifungal bioassay after complete characteristic of synthesized compounds.
Material and Methods
All the solvents and reagents were used as such as supplied from Merck (Darmstadt, Germany), S.D. Fine-Chem (Mumbai, India). FTIR spectra were recorded in KBr on a Schimadzu FTIR 8401 spectrometer. 1H-NMR and 13C-NMR spectra were recorded on a Bruker DRX 300 spectrometer operating at 300 MHz. The ESI mass spectra were measured on waters UPLC-TDQ spectrometer. The morphology of the catalyst was studied by high resolution electron microscopy HRTEM-300 KV Technai G2 30 S TWIN with gold coating equipped with energy dispersive X-ray spectroscopy.
General procedure for the synthesis of compounds (4-6)
Compounds 4-6 were synthesized by refluxing 3,3'-(1,4-phenylene)bis(1-phenylprop-2-en-1-one) 3 (1.0 mmol) with bis-nucleophile (2.0 mmol) in presence of 10 mmol % of MgFe2O4 MNP’s as catalyst for 200 min. On completion of reaction the catalyst was separated by using external magnet and the solvent was evaporated up to the dryness under reduced pressure to obtain a crude solid product. The solid obtained was stirred in water, filtered and recrystallized from EtOAc: Hexane solutions.
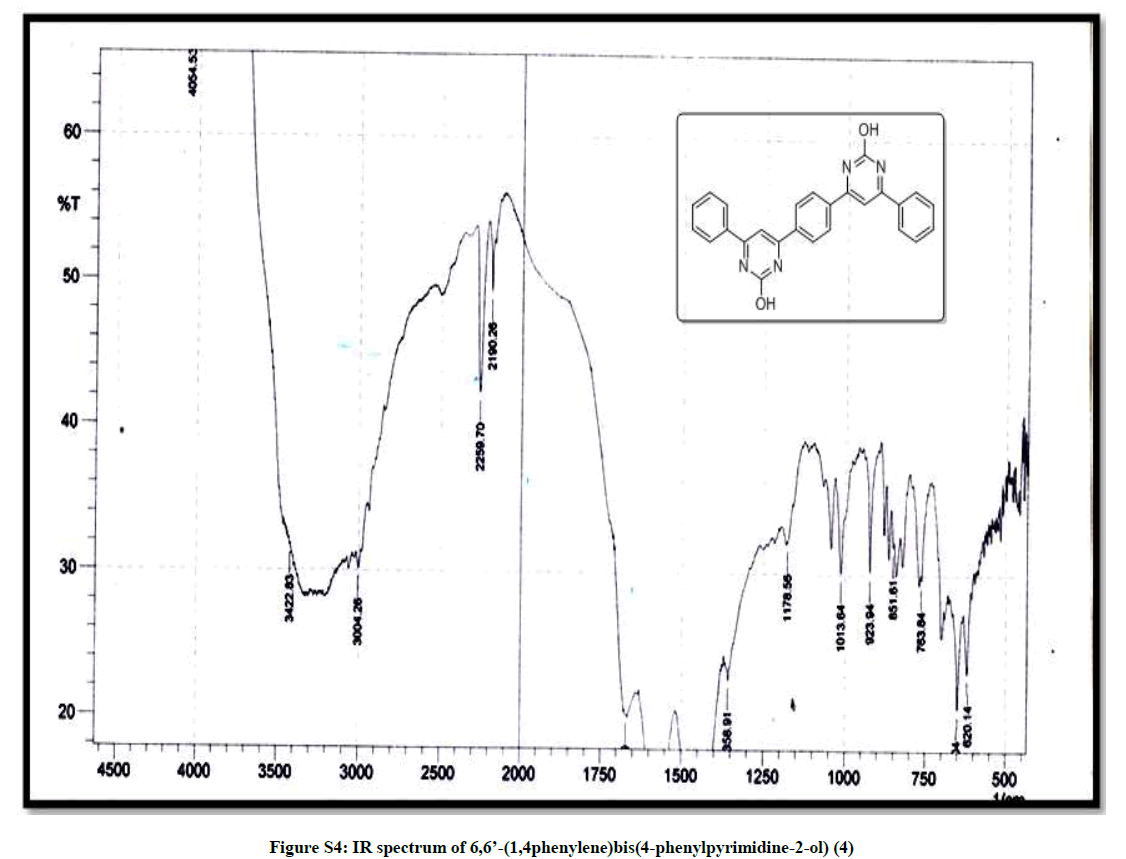
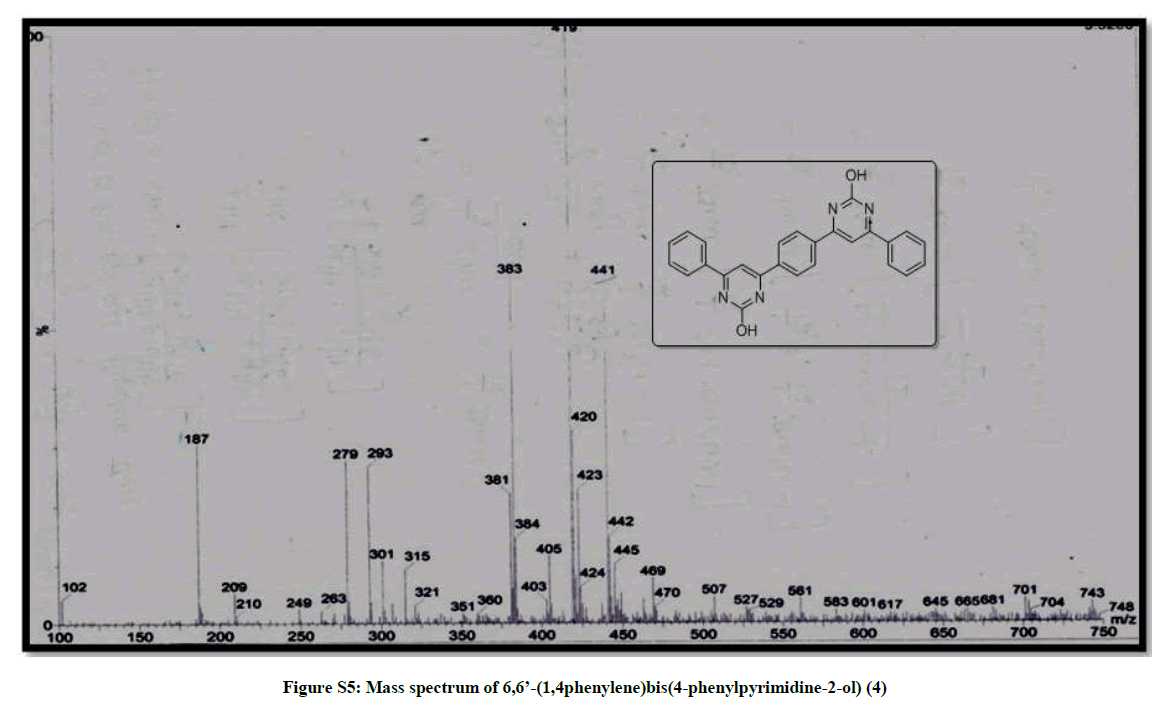
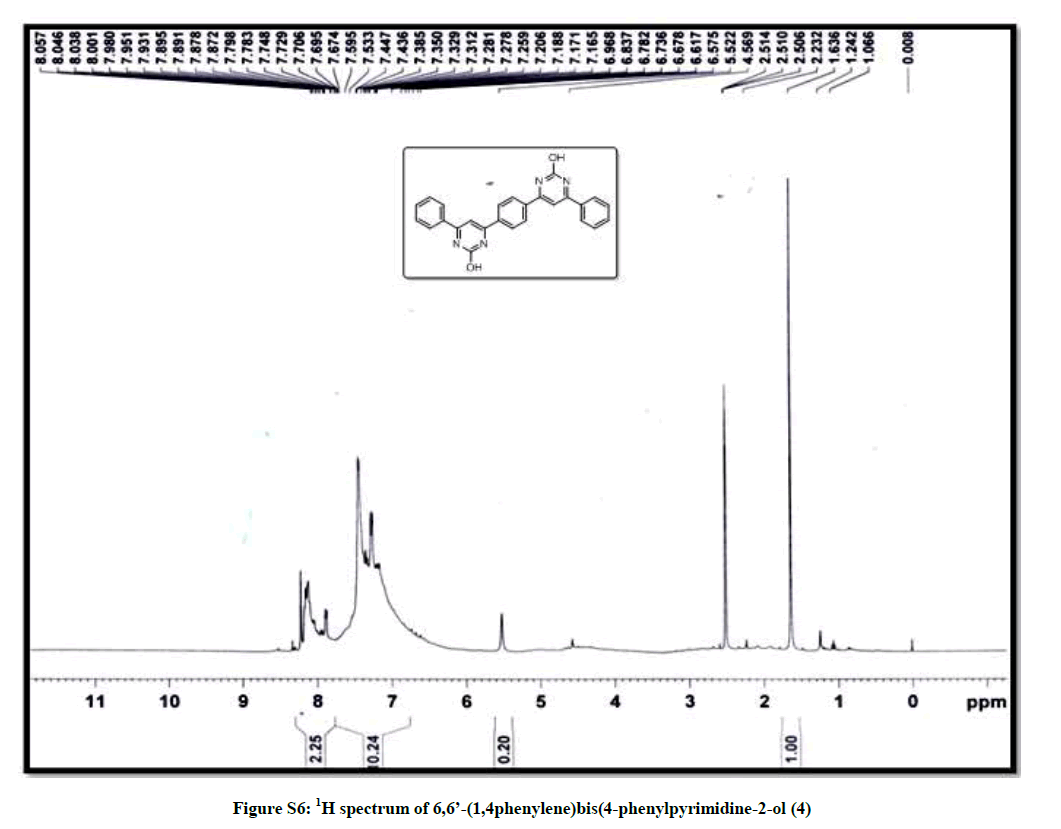
(6,6’-(1,4phenylene)bis(4-phenylpyrimidine-2-ol)) (4): Red orange solid, mp >300°C, Yield=86%; Rf=0.43 (2:8 EtOAc/hexane); IR (cm-1): 3422, 3004, 2259, 2190, 1178, 1013, 923; 1H-NMR (300 MHz, CDCl3): 7.79-7.67 (m, 1H), 7.38-7.17 (m, 6H); 13C-NMR (75 MHz, CDCl3): 174.84, 129.29, 128.98, 128.21, 126.99, 126.80; Mass ESI m/z (%) M++1=419. Anal. calcd. for C26H18N4O2: C, 74.63; H, 4.34; N, 13.39. Found: C, 74.78; H, 4.24; N, 13.45.
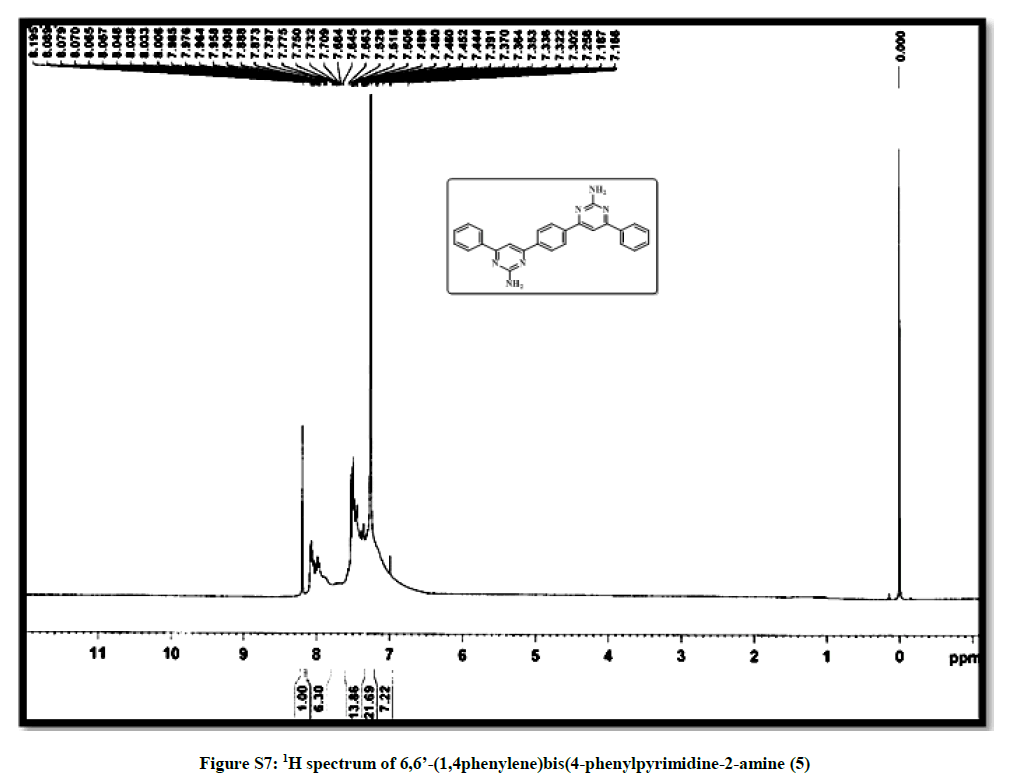
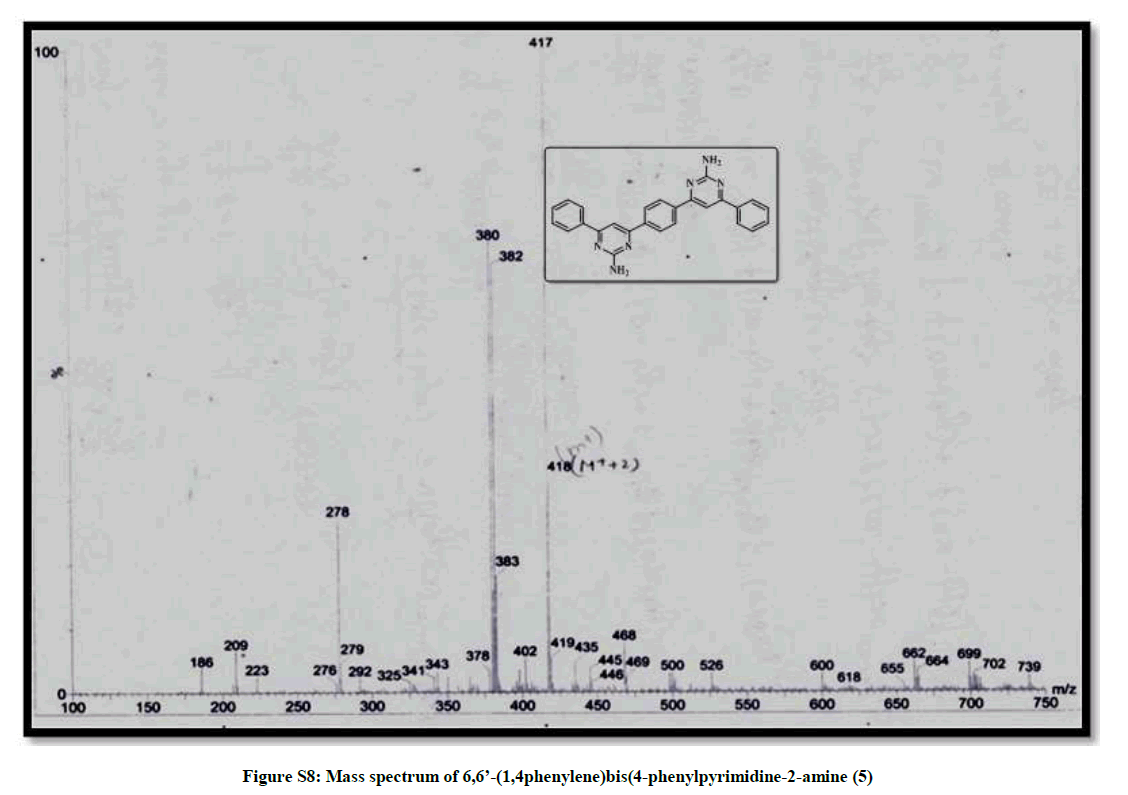
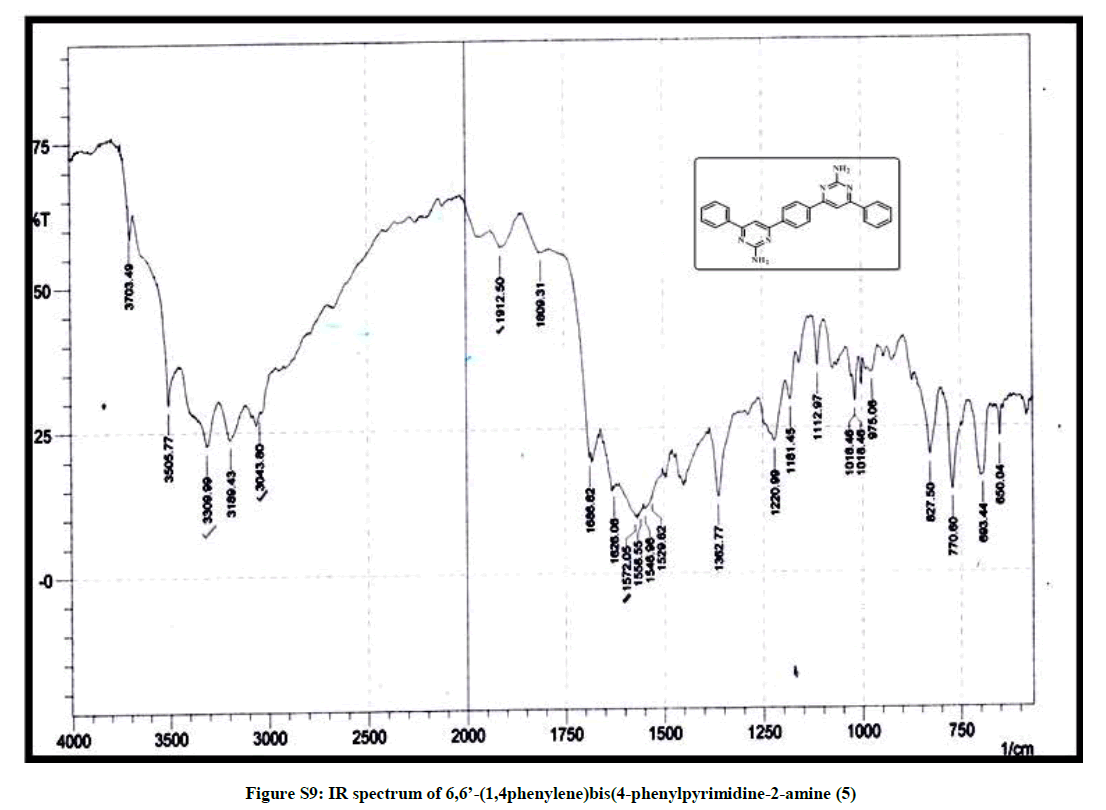
6,6’-(1,4-phenylene)bis(4-phenylpyridine-2-amine) (5): Bright yellow crystal, m.p. 220-221°C, Yield=85%, Rf=0.42 (2:8 EtOAc/hexane); IR (cm-1): 3309, 3043, 1912, 1572; 1H-NMR (300 MHz, CDCl3): 8.09 (s, 1H) 7.46-7.39 (m, 5H); 7.12-6.75 (m, 2H). Mass ESI m/z (%) M++1=417, M++2=418. Anal. calcd. for C26H20N6: C, 74.98; H, 4.84; N, 20.18. Found: C, 74.76; H, 4.72; N, 20.24.
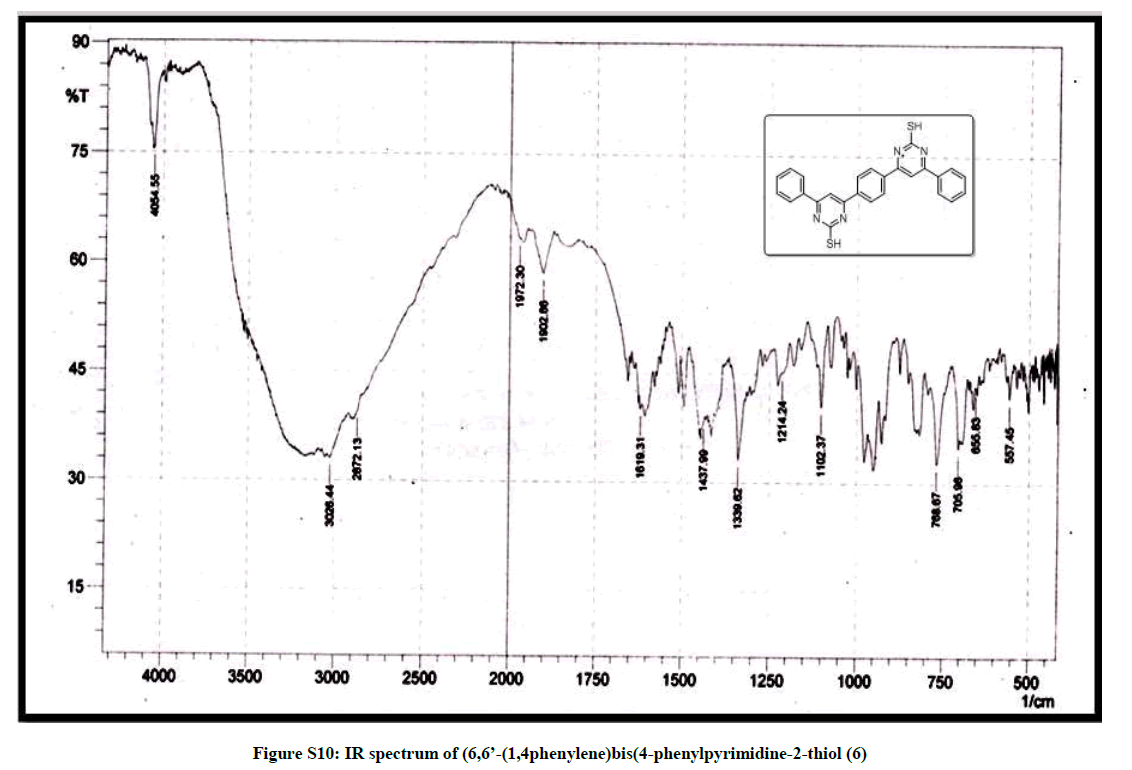
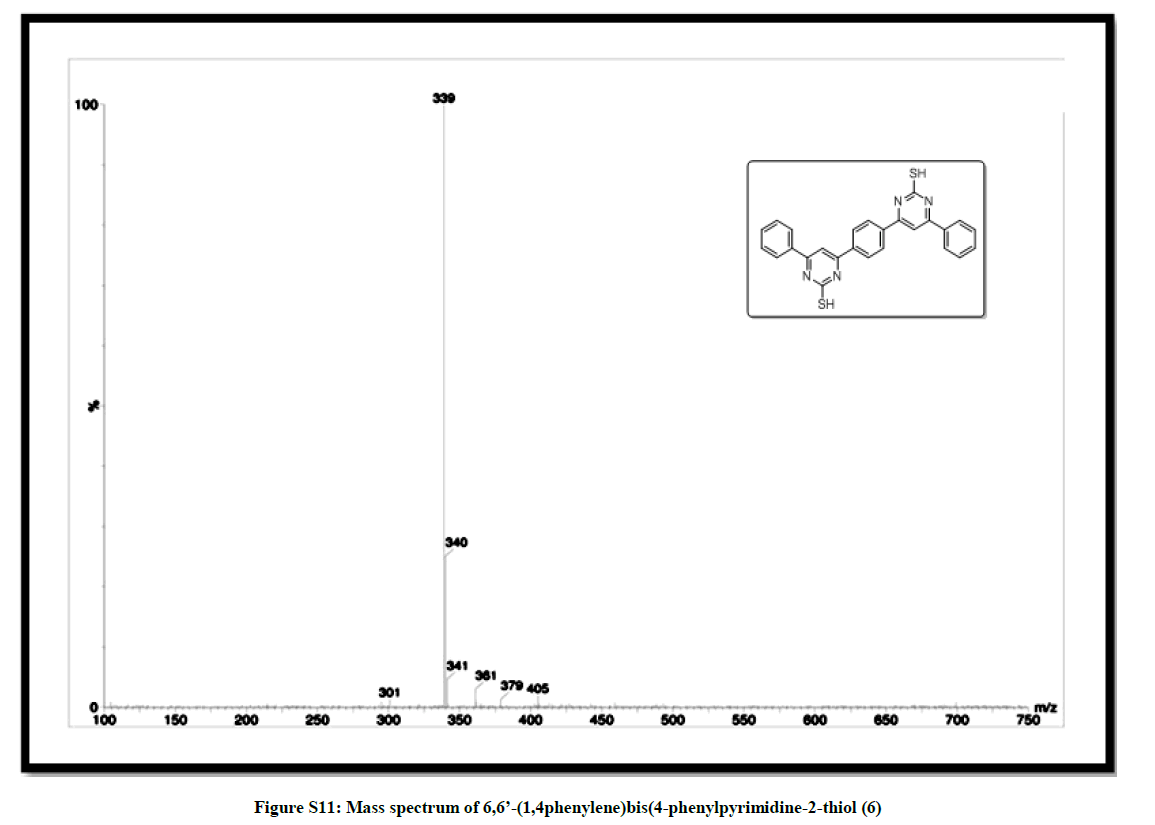
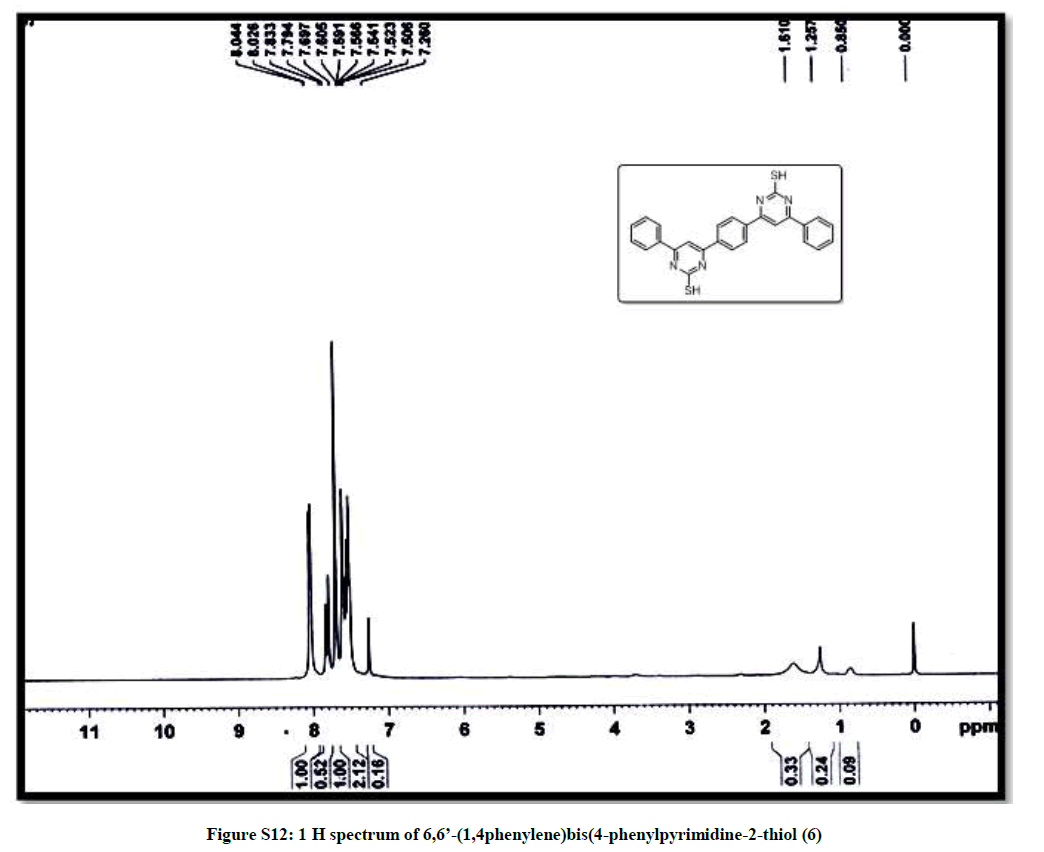
(6,6’-(1,4phenylene)bis(4-phenylpyrimidine-2-thiol)) (6): Pink solid, mp 270-72°C; Yield=90%; Rf=0.47 (2:8 EtOAc/hexane); IR (cm-1): 3026, 2827, 1972, 1619, 1437, 1339, 1214, 1102; 1H-NMR (300 MHz, CDCl3): 8.03 (d, J=5.4, 1H); 7.83-7.50 (m, 6H); 13C-NMR (75 MHz, CDCl3): 190.51, 143.78, 138.29, 137.12, 133.18, 129.19, 128.92, 128.75, 123.32. Mass ESI m/z (%) M+- phenyl)=339. Anal. calcd. for C26H18N4S2: C, 69.31; H, 4.03; N, 12.43; S, 14.23. Found: C, 69.43; H, 4.10; N, 12.33.
Results and Discussion
The targeted novel 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-ol) 4, 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-amine) 5 and (6,6'- (1,4-phenylene)bis(4-phenylpyrimidin-2-thiol) 6 were synthesized by treating MgFe2O4 (10 mmol%) as catalyst, precursor chalcone (1 mmol), 3 bifunctional nucleophiles (2 mmol) like urea, guanidine nitrate and thiourea at refluxing ethanol condition for the appropriate time period. The MgFe2O4 heterogeneous catalyst was easily removed by using simple external magnet. The products were obtained in 85-90% yields. The chalcone precursor was prepared by Claisen-Schmidt condensation [12] reaction condition (Scheme 1). MgFe2O4 nano catalyst can be very easily separated from the reaction mixture by applying external magnetic field owing to the super magnetic nature of Fe3O4 nanoparticles at room temperature.
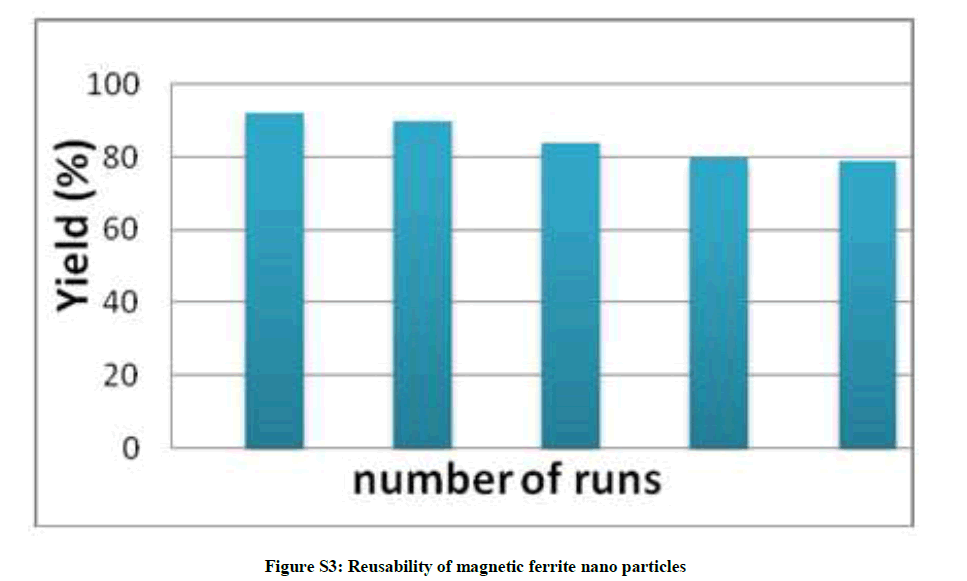
Reusability of magnetic ferrite nano particles were investigated by repetitive use of recovered catalyst. It was found that catalyst showed no appreciable change in activity even after five runs. Looking for the advantages of our developed strategy, we compared our protocol with various published reports [13] and it has been found that the catalyst reduced the reaction time drastically from 2-12 h (In the reported process) to 30-120 min for all the reaction to obtain the product in good yields.
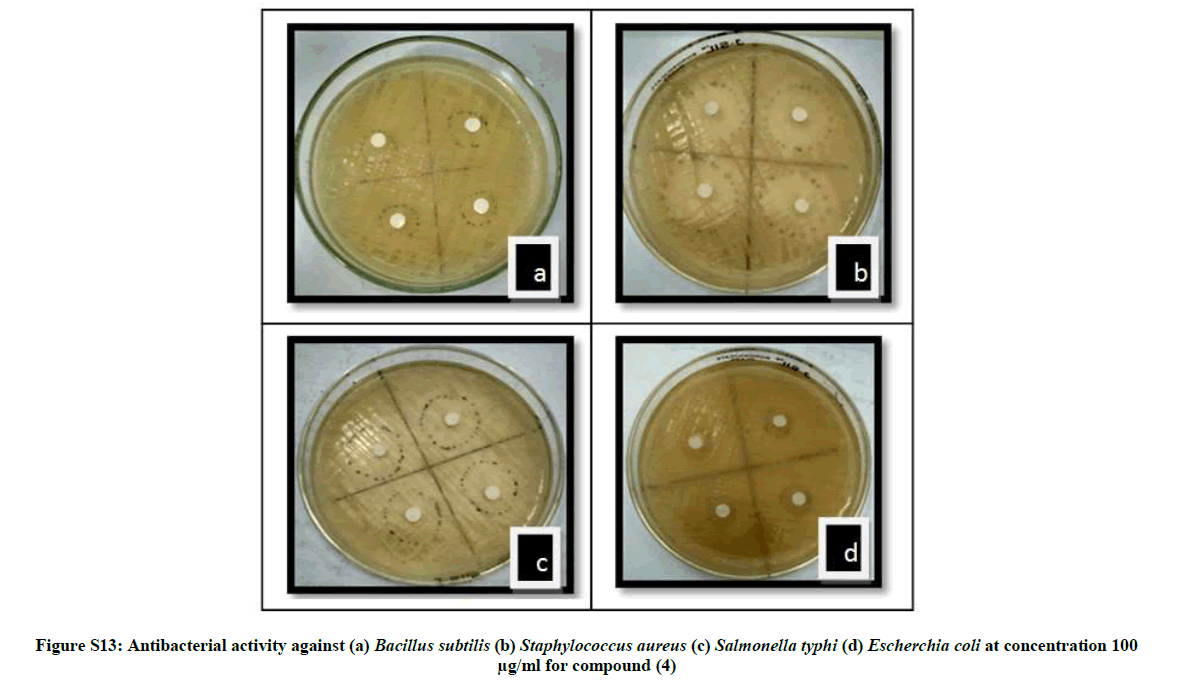
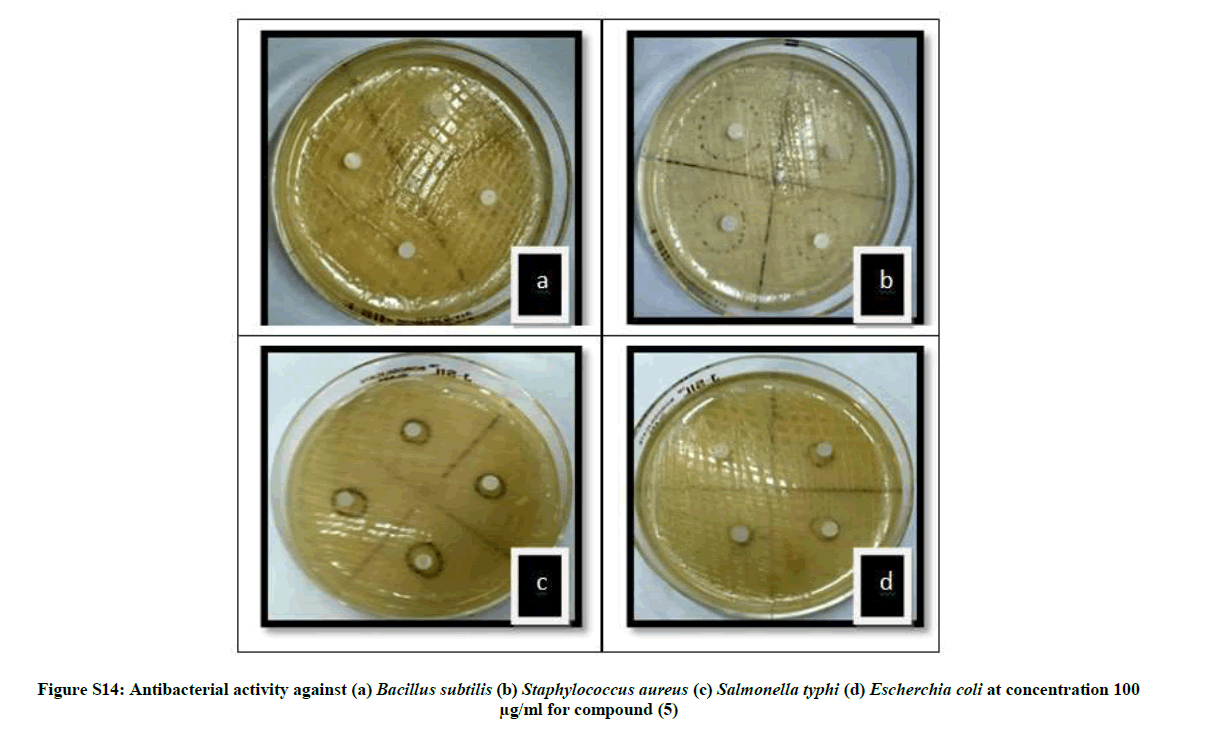
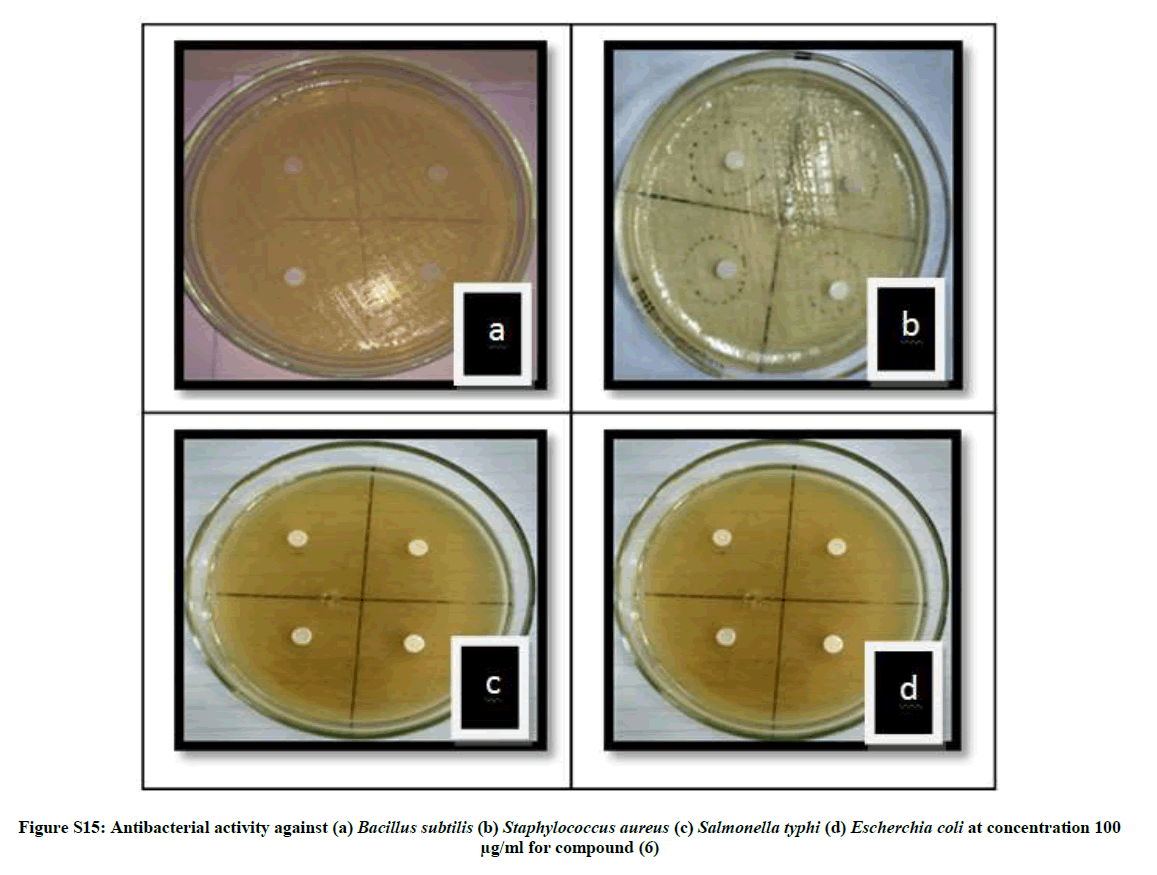
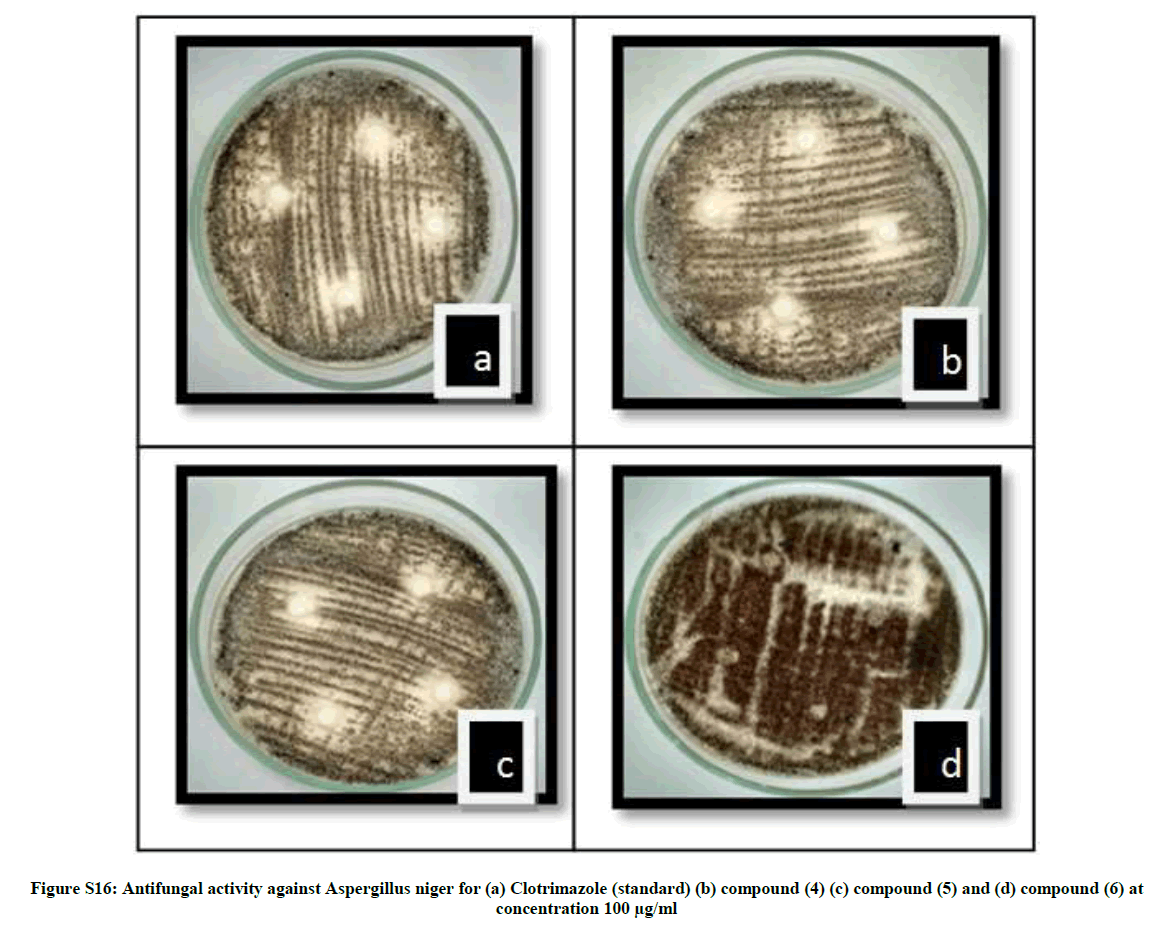
The antibacterial activity of newly synthesized compounds (4-6) was performed against Bacillus subtilis (MTCC No.121), Escherichia coli (MTCC No.118), Staphylococcus aureus (MTCC No. 96) and Salmonella typhi (MTCC No. 98) bacteria while antifungal activities was performed against Aspergillus niger (MTCC No. 281) and Candida albicans (MTCC No. 2479) using disc diffusion method Table 1.
| Zone of inhibition ± S.D. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Antibacterial activity | Compounds | Antifungal Activity | ||||||
| Concnentration | Bacillus subtilis | Staphylococcus aureus | Salmonella typhi | Escherchia coli | Concentration | Aspergillus niger | Candidia albicans | ||
| 100 µg/ml | 3.12 ± 0.47 | 3.37 ± 0.62 | 2.87 ± 0.47 | 3.62 ± 0.85 | 100 µg/ml | 6.50 ± 0.40 | 5.02 ± 0.45 | ||
| 200 µg/ml | 4.12 ± 0.47 | 4.12 ± 0.47 | 3.75 ± 0.86 | 3.37 ± 1.93 | 200 µg/ml | 8.75 ± 0.28 | 6.15 ± 0.78 | ||
| 4 | 300 µg/ml | 4.62 ± 0.85 | 5.0 ± 0.57 | 2.87 ± 0.47 | 3.87 ± 0.47 | 4 | 300 µg/ml | 12.87 ± 0.47 | 8.66 ± 0.85 |
| 400 µg/m | 4.75 ± 0.86 | 4.12 ± 0.47 | 3.25 ± 0.28 | 4.12 ± 0.47 | 400 µg/m | 14.12 ± 0.47 | 11.02 ± 0.87 | ||
| 100 µg/ml | 7.63 ± 0.62 | 19.25 ± 0.64 | 16.75 ± 0.95 | 8.00 ± 0.91 | 100 µg/ml | 3.37 ± 0.62 | 1.80 ± 0.12 | ||
| 200 µg/ml | 9.62 ± 2.17 | 19.87 ± 0.75 | 19.87 ± 0.47 | 8.75 ± 0.64 | 200 µg/ml | 4.37 ± 0.25 | 2.14 ± 0.15 | ||
| 5 | 300 µg/ml | 10.00 ± 1.29 | 23.00 ± 0.57 | 20.25 ± 0.50 | 10.75 ± 2.16 | 5 | 300 µg/ml | 5.40 ± 0.27 | 4.89 ± 0.79 |
| 400 µg/ml | 14.80 ± 1.31 | 21.12 ± 0.75 | 21.00 ± 0.57 | 14.62 ± 1.88 | 400 µg/ml | 7.00 ± 0.40 | 5.45 ± 0.48 | ||
| 100 µg/ml | 3.25 ± 0.50 | 2.57 ± 0.47 | 3.25 ± 0.50 | 3.00 ± 0.57 | 100 µg/ml | 3.07 ± 0.56 | 2.06 ± 0.45 | ||
| 200 µg/ml | 3.75 ± 0.5 | 3.87 ± 0.47 | 4.00 ± 0.57 | 3.12 ± 0.75 | 200 µg/ml | 4.37 ± 0.47 | 3.89 ± 0.87 | ||
| 6 | 300 µg/ml | 3.87 ± 0.47 | 4.87 ± 0.47 | 4.87 ± 0.47 | 3.37 ± 0.25 | 6 | 300 µg/ml | 5.87 ± 0.47 | 4.23 ± 0.48 |
| 400 µg/ml | 4.12 ± 0.47 | 6.0 ± 0.57 | 6.87 ± 0.47 | 3.62 ± 0.25 | 400 µg/ml | 6.37 ± 0.62 | 6.12 ± 0.45 | ||
| Control (DMSO)* | *** | *** | *** | *** | Control (DMSO)* | *** | *** | ||
| Ciprofloxacin | 100 µg/ml | 10.03 ± 0.75 | 20.00 ± 0.89 | 18.25 ± 0.98 | 9.04 ± 0.45 | Clotrimazoleb | 100 µg/ml | 7.50 ± 0.89 | 5.12 ± 0.45 |
Note: Concn. of the compounds were 10 mg/ml in DMSO; aStandard drug for antibacterial activity, bStandard drug for antifungal activity, *DMSO was taken as control
Table 1: Antibacterial and antifungal activity result for compound (4-6)
The average diameter of the zone of inhibition in the range of 2.57-19.87 mm indicates significant antimicrobial activities. When compared with ciprofloxacin compound 5 showed comparable antibacterial activities while 4 and 6 showed moderate activities. The order of antimicrobial activity among the compounds is 5>4>6 whereas the average zones of inhibition in the range of 1.80-6.50 indicates significant antifungal activity for the same compounds as compared to that of clotrimazole with inhibition zone (5.12-7.5 mm). Compound 4 showed high antifungal activity while compound 5 and 6 showed moderate activity. The order of antifungal activities among the compounds is 4>5>6. The minimum inhibition conc. (MIC) was also determined for all the newly synthesized compounds. The values of compounds 4-6 are depicted in Table 2.
| MIC (µg/ml) Compounds | |||
|---|---|---|---|
| Bacteria | 4 | 5 | 6 |
| Bacillus subtilis | 1.92 | 3.89 | 1.8 |
| Staphylococcus aureus | 1.79 | 9.82 | 1.35 |
| Salmonella typhi | 1.69 | 8.42 | 1.69 |
| Escherchia coli | 1.95 | 4.19 | 1.59 |
Table 2: Minimum Inhibition Concentration (MIC) of compounds (4-6)
S. aureus showed the best MIC for compound 5 whereas compound 6 gave the lowest MIC. The study of cytotoxicity for the compounds 4-6 on living tissues is also under investigation results for these studies may be provided in our further reports.
Conclusion
We have synthesized 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-ol) 4, 6,6'-(1,4-phenylene)bis(4-phenylpyrimidin-2-amine) 5 and (6,6'- (1,4-phenylene)bis(4-phenylpyrimidin-2-thiol) 6 by using cheaper heterogeneous catalyst working in milder reaction conditions, shorter reaction time, simple and cleaner workup procedure. The antimicrobial activities of these compounds showed that compound 5 exhibited comparable antibacterial activity with that of ciprofloxacin. While compound 4 showed antifungal activities comparable to clotrimazole. Biological potential of compounds 4 & 5 being as good as clotrimazole and ciprofloxacin respectively, can open plethora of opportunities in the field of research in antibiotics. Cytotoxicity for the compounds 4-6 is under investigation in our laboratory.
Acknowledgement
We thank the Institute of Pharmaceutical research of GLAU for all the biological work done and also GLAU providing for financial assistance and infrastructural support.
References
- J.U. Yuhong, R.S. Varma, J. Org. Chem., 2006, 71, 135-141.
- S. Sridhar, Y.R. Prasad, S.C. Dinda, Int. J. Pharm. Sci. Res., 2011, 2(10), 2562-2565.
- C.V. Rao, M. Suresh, P. Lavanya, K.N. Raju, S.B. Jonnalagadda, Org. Commun., 2011, 4(2), 33-41.
- S.G. Khanage, A. Raju, P.B. Mohite, R.B. Pandhare, Advanced Pharmaceutical Bulletin., 2012, 2(2), 213-222.
- K.M.H. Hilmy, M.D. Elsafty, A.R.I. Morsy, G.M.E. Aly, S.M. El-kousy, J. Am. Sc., 2011, 7(8), 308-314.
- S.R. Dhongade, V.A. Divate, S. Poonam, National Conference on Drug Designing and Discovery., 2013, 6(7), 70-73.
- S.A. Rahaman, Y.R. Pasad, P. Kumar, B. Kumar, Saudi Pharm. J., 2009, 17, 255-258.
- H.W. Lee, Y.K. Bok, B.A. Joong, Eur. J. Med. Chem., 2005, 40, 862-874.
- P.F. Juby, T.W. Hudyma, M. Brown, J.M. Essery, R.A. Partyka, J. Med. Chem., 1979, 22, 263-269.
- P.A.S. Smith, R.O. Kan, J. Org. Chem., 1964, 29, 2261-2265.
- K.M. Amin, F.M. Awadalla, A.A.M. Eissa, S.M. Abou-Seri, G.S. Hassan, Bioorg. Med. Chem., 2011, 19, 6087-6097.
- J.P. Singh, M. Dulawat, N. Jaitawat, S.S. Chundawat, A. Devpura, S.S. Dulawat, Indian J. Chem.,B2012, 51 1623-1627.
- A.A. Ghoneim, A.F. El-Farargy, Org. Chem. Curr. Res., 2016, 5, 1.
Supplementary Information
| S. No. | Content | Page no. |
|---|---|---|
| 1 | Preparation of the Magnesium Ferrite MNPs | P3 |
| 2 | Characterization of catalyst | P3 |
| 3 | X-ray diffraction pattern and HRTEM image of MgFe2O4 MNP's (Figure S1) | P3 |
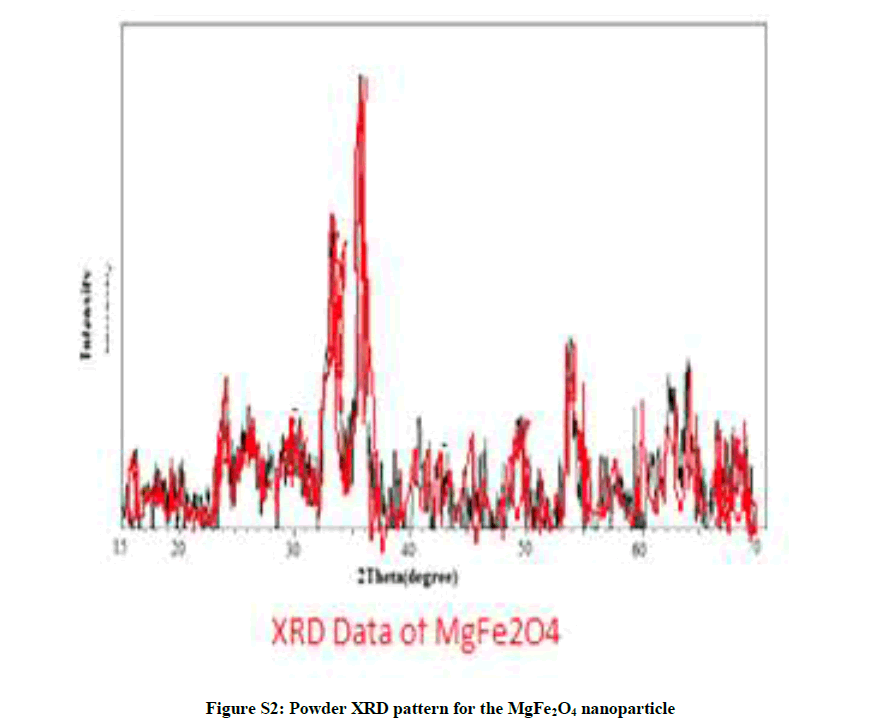
| 4 | Powder XRD pattern for the MgFe2O4 nanoparticle (Figure S2) | P4 |
| 5 | Reusability of magnetic ferrite nano particles (Figure S3) | P4 |
| 6 | Culture Media for Antimicrobial activity | P4 |
| 7 | Culture Media for Antimicrobial activity | P5 |
| 8 | IR & Mass spectrum of 6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-ol) 4 | P6 |
| 9 | 1H spectrum of 6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-ol 4 | P7 |
| 10 | IR & Mass spectrum of 6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-amine 5 | P8 |
| 11 | 1H spectrum of 6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-amine 5 | P9 |
| 12 | IR & Mass spectrum of (6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-thiol 6 | P10 |
| 13 | 1H spectrum of 6,6’- (1,4phenylene)bis (4-phenylpyrimidine-2-thiol 6 | P11 |
| 14 | Antibacterial activity against (a) Bacillus subtilis (b) Staphylococcus aureus (c) Salmonella typhi (d) Escherchia coli at concentration 100 µg/ml for compound (4) | P12 |
| 15 | Antibacterial activity against (a) Bacillus subtilis (b) Staphylococcus aureus (c) Salmonella typhi (d) Escherchia coli at concentration 100 µg/ml for compound (5) | P12 |
| 16 | Antibacterial activity against (a) Bacillus subtilis (b) Staphylococcus aureus (c) Salmonella typhi (d) Escherchia coli at concentration 100 µg/ml for compound (6) | P13 |
| 17 | Antifungal activity against Aspergillus niger for (a) Clotrimazole (standard) (b) compound (4) (c) compound (5) and (d) compound (6) at concentration 100 µg/mL | P13 |
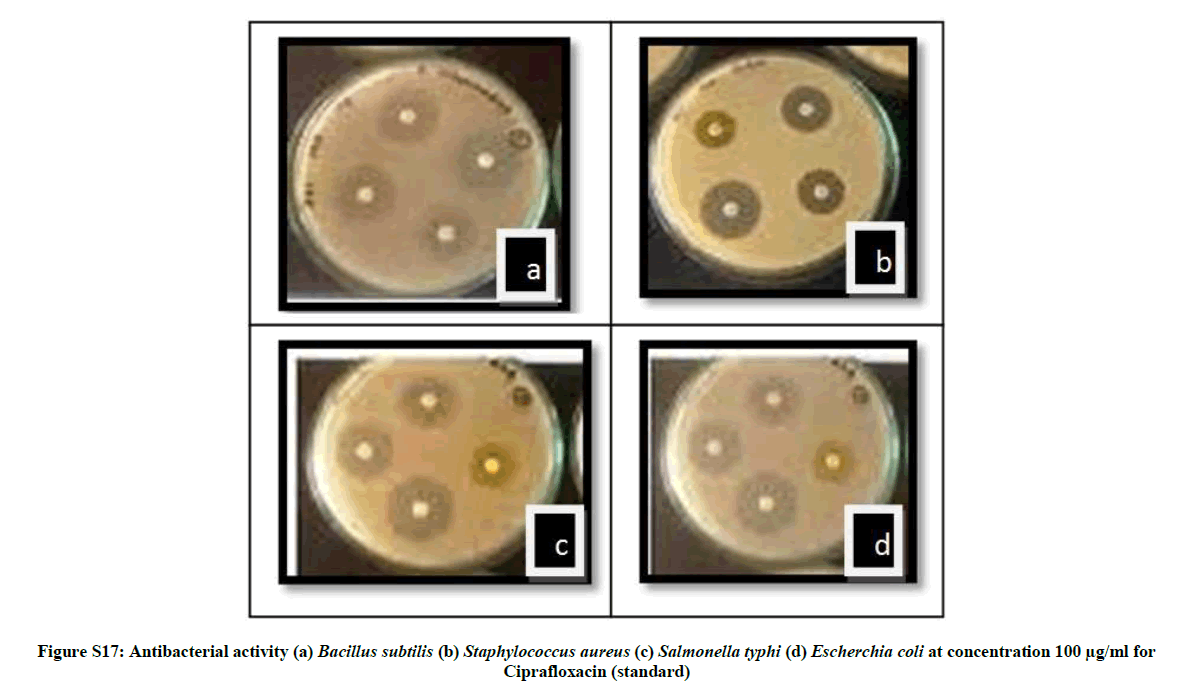
| 18 | Antibacterial activity against ( (a) Bacillus subtilis (b) Staphylococcus aureus (c) Salmonella typhi (d) Escherchia coli at concentration 100 µg/ml for Ciprafloxacin (standard) | P14 |
Preparation of the magnesium ferrite MNPs
Nanoparticle of MgFe2O4 was synthesized by combustion technique. 2.0 mmol of Fe(NO3)3·9H2O and 1.0 mmol of MgCO3 dissolved in HNO3 and mixed uniformly. 1.0 mmol of monoethanolamine was added to the reaction mixture followed by 1.0 mmol of each of sucrose and excess of nitric acid respectively. The resulting mixture was then put on a hot plate at 80°C till it completely dried to a black residue. This black residue was then kept in muffle furnace at 600°C for 6-8 h to obtain nanoparticle of MgFe2O4.
Characterization of catalyst
The high resolution electron microscopy (HRTEM) of NP’S is shown in Figure S1. The particle size of the magnesium ferrite NP’s sample is typically in the range of 100-200 nm. The small crystallize are irregular in shape and are attached to each other along the grain boundaries. The material was verified by XRD data which matched very well with standard data. Size of crystallite was found by 100 nm from analysis of XRD profile by Debye Sheerer equation surface area was found to be 12 m2/gm Figure S2.
Culture media for antimicrobial activity
Culture media for antimicrobial activity was performed by taking nutrient agar-agar media (15 g), peptone (5 g), yeast extract (2 g), Beef extract (1 g) (which dissolved in distilled water with the application of heat), pH of the solution was adjust to 6.5-7.0 with the help of 0.1%w/v sodium chloride solution. The culture media, disc and glass wares was sterilized by autoclaving at 15 lb/sq.inch pressure for 15 minutes and the synthesized compounds were tested for antimicrobial activity (Figures S3-S17).
Culture Media for Antimicrobial activity
Culture media for antifungal activity was performed by taking nutrient agar-agar media (15 g), peptone (10 g), glucose (40 g) (dissolved in distilled water with the application of heat), pH of the solution was adjust to 6.5-7.0 with the help of 0.1%w/v sodium chloride solution. The culture media, disc and glass wares was sterilized by autoclaving at 15 lb/sq. inch pressure for 15 min and the synthesized compounds were tested for antifungal activity.
Characterization of synthesized compounds
Antimicrobial activities