Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
A Facile and an Efficient Synthesis of Baylis-Hilman Adducts via Vinylalumination of Isatins
Rajitha Galla1, Sasikala Maadwar1,2* and Arupula SanjeevaKumar2
1Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Vishwavidhyalayam, Tirupathi, India
2Medicinal Chemistry and Pharmacology Division, Indian Institute of Chemical Technology, Hyderabad, India
- *Corresponding Author:
- Sasikala Maadwar
Institute of Pharmaceutical Technology
Sri Padmavathi Mahila Vishwavidhyalayam, Tirupathi, India
Abstract
A highly efficient synthesis of Baylis-Hilman derivatives has been achieved via a vinylalumination of isatins at room temperature. The resulting oxindole scaffolds were obtained in moderate to high yields. The main advantages of this manuscript are short reaction time, operationally simple, purification of products by non-chromatographic method.
Keywords
2-nitroalkanoic acid, Isatin, Grinding, Solvent-free condition, Oxindole.
Introduction
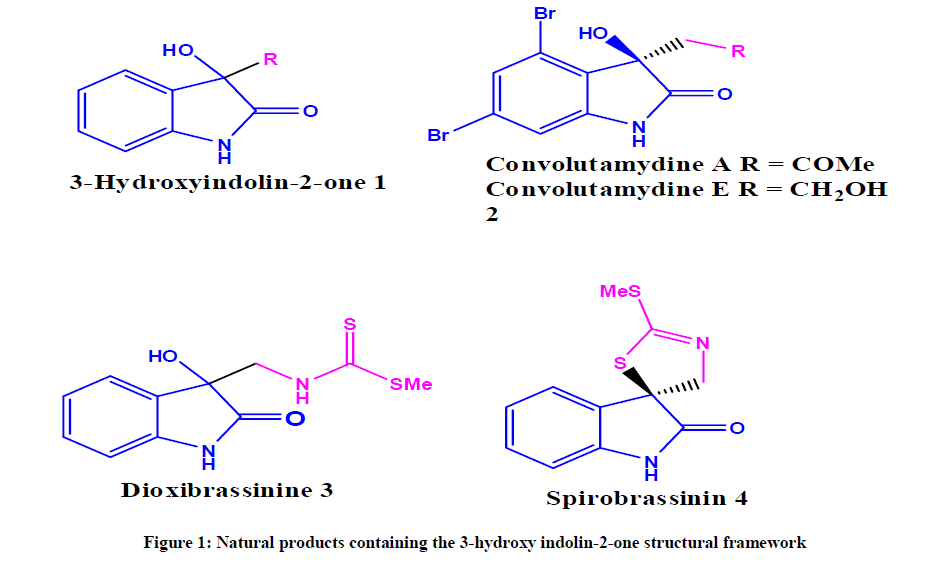
C3-functionalized oxindoles are privileged structural motifs and are present in a variety natural products and biologically active compounds [1]. In particular, 3-substituted-3-hydroxy-2-oxindoles were present in a number of bioactive compounds such as, welwitindolinone C, TMC-95s, convolutamydines, celogentin K, and SM-130686 (Figure 1) [2]. Owing to their broad range of biological activities, several methods have been reported for the synthesis of hydroxyl oxindole scaaffolds [3] such as, nucleophilic addition to isatins [4], hydroxylation of 3-substituted oxindoles [5] and intramolecular arylation reactions [6].
Organo aluminum intermediates have been well studied in organic chemistry.
1. Hydroalumination of acetylenes to prepare vinylaluminum intermediates for application in organic syntheses has been known for decades.
2. In 1987, Tsuda, Saegusa, and co-workers reported the preparation of novel vinylaluminum reagents, [7] (alkoxycarbonyl)vinyl]diisobutylaluminum and aluminum allenoates by the hydroalumination of R, â-acetylenic carbonyl compounds with diisobutylaluminum hydride (DIBAL-H) in the presence of hexamethylphosphoric amide (HMPA).
3. They treated these aluminum intermediates with protic acid, allyl bromide, and carbonyl compounds.
4. The reaction with carbonyl compounds provides functionalized allyl alcohols.
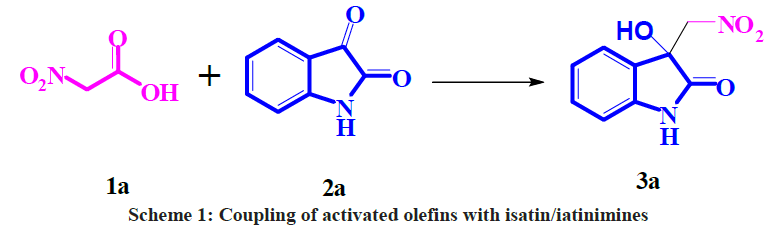
The coupling reaction of an un-activated π – partner with isatins has attracted because this type of protocol provides an easy way to synthesis of Baylis-Hilman adducts and it could defeats the negative aspects of the Baylis-Hilman reaction [8]. In our previous communications, we have reported reductive coupling of activated olefins with isatin/iatinimines (Scheme 1) [9]. As a part of ongoing research from our laboratory in the synthesis of 3-substituted-3-hydroxy-2-oxindole [10,11], in this paper, we would like to report a facile and an efficient synthesis of Baylis- Hilman adducts through the vinyl alumination at room temperature in THF.
Materials and Methods
Experimental part
General
All reactions were carried out without any special precautions in an atmosphere of air. Chemicals were purchased from Fluka and S. D. Fine Chemicals. TLC: precoated silica gel plates (60 F254, 0.2 mm layer; E. Merk 1H-NMR Spectra: Varian 200 or Bruker 300 spectrometer; in CDCl3; δ in ppm, J in Hz. Mass spectra: VG Autospec; in m/z [12-14].
General procedure
To a solution of bromonitro alkane 2 (2 mmol) in a mixture of solvent (THF: H2O: 9: 1, 10 ml), zinc powder (2 mmol) and ammonium chloride (1 mol%) was added and mixture stirred for 5 minutes. Then isatins (1 mmol) were added and stirred for stipulated time (Table 1). The reaction is monitored by TLC. After completion of reaction, the mixture was extracted with ethyl acetate (3X5 ml). The combined organic layer washed with brine solution, dried over Na2SO4, and concentrated under reduced pressure (rotary evaporator). The crude products were purified by silica gel column chromatography using ethylacetate/hexanes (3: 7). All compounds were characterised by (M.P., NMR, Mass and IR) spectral data.
| Entry | base (mol) | time | Yield (%) |
|---|---|---|---|
| 1 | K2CO3 (5) | 20 | 30 |
| 2 | Na2CO3 (5) | 20 | 30 |
| 3 | KOH (3) | 12 | 75 |
| 4 | KOH (5) | 8 | 80 |
| 5 | NaOH (3) | 10 | 90 |
| 6 | NaOH (6) | 5 | 97 |
| 7 | NaOH (7) | 5 | 96 |
| 8 | NaHCO3 (5) | 30 | 20 |
| 9 | --- | 30 | --- |
aReaction conditions: Isatin (1equiv.), 2-nitroalkanoic acid (2 equiv.), base (X equiv.) at rt
Table 1: Screened bases for the decarboxylative coupling of 2-nitroacetic acid with isatin
To a solution of nitro alkanoic acid 2 (2 mmol) and isatin 1 (1 mmol) was added NaOH (6 mmol) and stirred for stipulated time (Table 1). The reaction is monitored by TLC. After completion of reaction, the mixture was extracted with ethyl acetate (3 X 5 ml). The combined organic layer washed with brine solution, dried over Na2SO4, and concentrated under reduced pressure (rotary evaporator). All compounds were characterised by (M.P., NMR, Mass and IR) spectral data [15].
Spectral data of compounds
1-Benzyl-5-bromo-3-hydroxy-3-(nitromethyl)indolin-2-one (Table 1, Entry-4): Pale yellow solid; M. p. 121-123°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.85 (d, J=15.8 Hz, 1H), 4.95 (d, J=15.8 Hz, 1H), 5.05 (s, 2H), 6.66 (d, J=8.3 Hz, 1H), 6.95 (s, 1H), 7.41-7.19 (m, 6H), 7.62 (s, 1H); 13C-NMR (70 MHz, CDCl3 + DMSO-d6) δ (ppm)=48.7, 77.7, 83.0, 101.0, 116.6, 120.0, 132.4, 132.7, 134.7, 135, 138.1, 140.4, 147.6, 179.2; IR (KBr) ν=3331, 2921, 1711, 1621, 1561, 1391, 1097, 757 cm-1; MS (ESI): m/z 399 (M+ Na).
3-Hydroxy-3-(nitromethyl)-1-phenylindolin-2-one (Table 1, Entry-5): White solid; M. p. 99-101°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.92-5.11 (m, 2H), 6.75 (d, J=7.7 Hz, 1H), 6.91 (s, 1H), 7.08 (t, J=7.4 Hz, 1H), 7.29 (t, J=7.5 Hz, 1H), 7.40-7.55 (m, 6H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=72.7, 78.4, 109.1, 122.3, 125.4, 126.4, 128.1, 128.4, 129.7, 133.8, 140.2, 143.7, 174.1; IR (KBr) ν= 3328, 2933, 1709, 1617, 1559, 1467, 1389, 761 cm-1; MS (ESI): m/z 307 (M+Na).
5-Fluoro-3-hydroxy-3-(nitromethyl)-1,3-dihydro-2H-indol-2-one (Table 1, Entry-6): White solid; M. p. 161-163°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.90-4.82 (m, 2H), 6.69 (s, 1H), 6.84-6.80 (m, 1H), 6.94 (dt, J=2.2, 9.0 Hz, 1H), 7.14 (d, J=7.7 Hz, 1H), 10.39 (s, 1H); 13C-NMR(75 MHz, CDCl3 + DMSO-d6) δ (ppm)=72.9, 78.0, 110.8, 112.7, 116.6, 129.7, 138.7, 159.4, 175.8; IR (KBr) ν=3358, 3271, 2924, 1726, 1612, 1555, 1470, 1374, 1182, 744 cm-1; MS (ESI): m/z 249 (M+Na).
3-Hydroxy-5-iodo-3-(nitromethyl)-1,3-dihydro-2Hindol-2-one (Table 1, Entry-9): White solid; M. p. 274- 276°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.91-4.81 (m, 2H), 6.66 (s, 1H), 6.70 (d, J=8.3 Hz, 1H), 7.53 (dd, J=1.7, 8.3 Hz, 1H), 7.64 (d, J=1.5 Hz, 1H), 10.46 (s, 1H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=72.6, 78.1, 84.2, 112.6, 130.5, 133.1, 138.6, 142.4, 175.2; IR (KBr) ν=3388, 3271, 2924, 1726, 1612, 1555, 1471, 1182, 822 cm-1; MS (ESI): m/z 357 (M+Na).
3-Hydroxy-3 (nitromethyl)-5-(trifluoromethoxy)indolin-2-one (Table 1, Entry-11): Pale yellow solid; Mp 115-117°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.88 (s, 2H), 6.76 (s, 1H), 6.90 (d, J=8.4 Hz, 1H), 7.10 (d, J=8.4 Hz, 1H), 7.30 (s, 1H ), 10.55 (s, 1H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=84.6, 91.4, 111.5, 113.4, 113.9, 121.7, 128.9, 134.7, 156.5, 179.6; IR (KBr) ν=3258, 3171, 2944, 1722, 1632, 1545, 1374, 1102, 748 cm-1; MS (ESI): m/z 315 (M+Na).
3-Hydroxy-4,6-Dibromo-3-(nitromethyl)-1,3-dihydro-2Hindol-2-one (Table 1, Entry-13): White solid; M. p. 195-197°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.95 (d, J=13.4 Hz, 1H), 5.30 (d, J=13.4 Hz, 1H), 6.75 (s, 1H ), 6.98 (s, 1H), 7.23 (s, 1H), 10.75 (s, 1H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=83.4, 87.7, 113.5, 122.0, 124.5, 126.6, 129.4, 144.2, 179.6; IR (KBr) ν=3356, 3178, 1714, 1610, 1556, 1373, 1064, 846 cm-1; MS (ESI): m/z 387 (M+H).
3-Hydroxy-5,7-Dibromo-3-(nitromethyl)-1,3-dihydro-2Hindol-2-one (Table 1, Entry-14): White solid; M. p. 163-165°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.90 (s, 2H), 6.83 (s, 1H), 7.47 (d, J=1.5 Hz, 1H), 7.53 (d, J=1.51 Hz, 1H), 10.75 (s, 1H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=84.4, 89.7, 105.5, 118.4, 127.5, 132.3, 133.4, 140.0, 179.6; IR (KBr) ν=3346, 3148, 1718, 1625, 1566, 1014, 856 cm-1; MS (ESI): m/z 387 (M+ H), (M+ 2).
3-Hydroxy-7-Bromo-3-(nitromethyl)-1,3-dihydro-2Hindol-2-one (Table 1, Entry-15): White solid; M. p. 136-138°C; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=4.90 (d, J=6.2 Hz, 2H), 6.76 (s, 1H), 6.90 (t, J = 7.7 Hz, 1H), 7.34 (d, J=11.3 Hz, 1H), 7.38 (d, J=11.8 Hz, 1H), 10.64 (s, 1H); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=73.3, 78.4, 102.4, 123.0, 123.1, 129.2, 132.7, 141.7, 175.4; IR (KBr) ν=3196, 1717, 1612, 1550, 1443, 777 cm-1. MS (ESI): m/z 308 (M+ Na).
4,7-Dichloro-2-methylene-3-(nitromethyl)indolin-3-one (Table 1, Entry-17): White Solid; M. p. 152-154°C; 1H-NMR (300 MHz, CDCl3) δ (ppm)=5.08 (d, J=13.2 Hz, 2H), 6.88 (s, 1H), 6.91 (d, J=8.4 Hz, 1H), 7.21 (d, J=8.4 Hz, 1H), 10.74 (s, 1H); 13C-NMR (75 MHz, CDCl3) δ (ppm)=84.0, 85.6, 125.4, 126.2, 128.8, 129.2, 132.8, 144.8, 179; IR (KBr) ν=3551, 3248, 2974, 1727, 1619, 1552, 1488, 1078, 512 cm-1; MS (ESI): m/z = 299 (M+ Na).
5-Bromo-3-hydroxy-3-(1-nitroethyl)indolin-2-one (Table 1, Entry-18): White solid; M. p. 167-169°C; diastereomeric ratio 70:30; 1H- NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=1.40 (d, J=6.9 Hz, 3H), 5.0 (q, 1H), 6.73 (s, 1H), 6.77-6.81 (m, 1H), 7.34-7.40 (m, 1H), 7.51 (s, 1H), 10.50 (s, 1H) (Major); 1.77 (d, J=6.9 Hz, 3H), 4.98 (q, 1H), 6.68 (s, 1H), 6.83-6.87 (m, 1H), 7.20-7.30 (m, 1H), 7.34 (s, 1H), 10.43 (s, 1H) (Minor); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=6.8, 89.6, 96.9, 118.8, 123.8, 129.4, 133.0, 133.2, 139.6, 179.2 (Major); 6.4, 88.9, 96.2, 118.2, 124.0, 128.8, 129.6, 133.4, 139.2, 178.8 (Minor); IR (KBr) ν=3356, 1729, 1628, 1572, 1558, 1487, 1371, 1161, 847 cm-1; MS (ESI): m/z 323 (M+Na).
5-Iodo-3-hydroxy-3-(1-nitroethyl)indolin-2-one (Table 1, Entry-19): White solid; M. p. 278-280°C; diastereomeric ratio 80:20; 1H-NMR (300 MHz, CDCl3 +DMSO-d6) δ (ppm)=1.40 (d, J=6.9 Hz, 3H), 4.50 (s, 1H) 4.96 (q, 1H), 6.70 (d, J=8.2 Hz, 1H), 7.55-7.60 (m, 1H), 7.68 (s, 1H), 10.451 (s, 1H) (Major); 1.77 (d, J = 6.9 Hz, 3H), 4.52 (s, 1H), 5.02 (q, 1H), 6.75 (d, J = 8.1 Hz, 1H), 7.57-7.62 (m, 2H), 10.38 (s, 1H) (Minor); 13C-NMR (75 MHz, CDCl3 +DMSO-d6) δ (ppm)=6.78, 89.6, 97.4, 110.7, 113.0, 115.4, 132.4, 136.5, 158.6, 179.4 (Major); 6.74, 89.4, 97.3, 110.2, 113.4, 115.0, 132.0, 136.7, 158.2, 179.4 (Minor); IR (KBr) ν=3381, 3248, 2934, 1733, 1614, 1552, 1472, 1188, 816 cm-1; MS (ESI): m/z 371(M+Na).
3-Hydroxy-3-(1-nitroethyl)indolin-2-one (Table 1, Entry-20): Pale yellow solid; M. p. 110-112°C; diastereomeric ratio 60:40; 1H-NMR (300 MHz, CDCl3 + DMSO-d6) δ (ppm)=1.35 (d, J=6.7 Hz, 3H), 4.0 (s, 1H), 4.95 (q, 1H), 6.80-7.10 (m, 3H), 7.18 (dd, J=2.4, 5.8 Hz, 1H), 10.46 (s, 1H) (Major); 1.74 (d, J = 6.7 Hz, 3H), 3.9 (s, 1H), 5.43 (q, 1H), 6.90-7.05 (m, 3H), 7.30-7.40 (m, 1H), 10.36 (s, 1H) (Minor); 13C-NMR (75 MHz, CDCl3 + DMSO-d6) δ (ppm)=7.2, 88.6, 98.4, 110.2, 123.6, 124.4, 126.8, 131.2, 141.0, 176.0(Major); 6.9, 89.2, 97.3, 109.0, 122.4, 123.8, 125.6, 131.0, 140.8, 176.4 (Minor); IR (KBr) ν=3250, 2906, 1717, 1630, 1552, 1480, 1313, 1088 cm-1; MS (ESI): m/z 245 (M+Na).
Results and Discussions
Initially, we focused on the screening of a base for the synthesis of 3-substituted 3-hydroxy oxindoles 3a by using the grinding reaction of 2-nitroacetic acid 2a (2 mmol) with isatin 1a (1 mmol) under solvent free conditions at room temperature and the results are depicted in Figure 2. It was observed that the reaction preceded efficiently using sodium hydroxide as base and resulted in high yield of desired product in very short reaction time (5 min). However, with other bases the reaction was comparatively slow and gave less yield of the product even after stretching the reaction time (2 h).
With the optimized conditions in hand, a variety of isatins were next explored to investigate generality of this novel reaction and the detailed results are summarised in Table 1. Further we have extended this protocol to different substituted isatins. For example, 5-halo isatins reacted with niroacetic acid 2a the standard reacton condition and resulted into high yields (Table 1, Entries 6-9). Other 5-substituted isatins also afforded comparative good yields of products (Table 1, Entries 10-12). In addition to this N-substituted isatins (Table 1, entry- 2, 3, 4, 5) were subjected to modified Henry reaction under the optimized conditions, the corresponding products are obtained in good yield. Similarly, 4-substitued, 7-substitued and also disubstitued isatins (Table 1, Entries 13-17) participated in the reaction and resulted into comparatively less yields. Then, we extended this protocol for nitopropanoic acid 2b which gave good yields. For example, simple isatin (Table 1, Entry 20) reacted with 2b gave high yields with 60:40 diastereoselectivity. However, the reaction of substituted isatins (Table 1, Entries 18-19) with bromonitroethane in identical conditions provided good yields of products with improved selectivity.
Conclusion
In summary, we have demonstrated a metal mediated an efficient method for the synthesis of functionalized 3-hydroxy-3-(nitromethyl)indolin-2- one derivatives in aqueous media. The present method provides a general protocol for the synthesis of variety of substituted and functionalized oxindoles of biologically interest.
Acknowledgement
We thank Mrs. Swetha and Mr. Devender, (Discovery Labs, CSIR-IICT, Hyderabad), for providing facilities to perform chemical reactions. We would like to acknowledge Mr. Reddy G (ddlabs.in, Hyderabad), for writing assistance and editorial support.
References
- S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20.
- Y. Kamano, H.P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama, G.R. Pettit, Tetrahedron Lett.,1995, 36, 2783.
- P. Hewawasam, N. Meanwell, A.; Gribkoff, V.K.; Dworetzky, S.I.; Biossard, C. G. Bioorg. Med. Chem. Lett.,1997, 7, 1255.
- P. Hewawasam, N.A. Meanwell, V.K. Gribkoff, U.S. Patent 5, 1997, 602, 169.
- R. Shintani, M. Inoue, T. Hayashi, Angew. Chem. Int. Ed., 2006, 45, 3353.
- Y. Hamashima, T. Suzuki, H. Takano, Y. Shimura, M. Sodeoka, J. Am. Chem. Soc., 2005, 127, 10164.
- S. Lee, J.F. Hartwig, J. Org. Chem., 2001, 66, 3402.
- V. Schulz, M. Davoust, M. Lemarie, J.F. Lohier, J. Sopkova de Oliveira Santos, P. Metzner, J.F. Briere, Org. Lett.,2007, 9, 1745.
- W.R. Conn, H.G. Lindwall, J. Am. Chem. Soc., 1936, 58, 1236.
- Lu Liu, Z. Shilei, X. Fei, G. Lou, Z. Haoyi, S. Ma, D. Wenhu, W. Wei, Chem. Eur. J., 2011, 17, 7791.
- H. Ren, G. Dunet, P. Mayer, P. Knochel, J. Am. Chem. Soc.,2007, 129, 5376.
- E. Oler, K. Reininger, U. Schmidt, Angew. Chem., Int.Ed. Engl., 1970, 9, 457.
- Z. Hui, D. Wei, Qing Xaing, Chine. Chem. Lett., 2005, 16, 1459.
- S. Yamamura, M. Toda, Y. Hirata, Organic Syntheses., 1988, 6, 289.
- H.M. Meshram, D.A Kumar, P.R. Goud, B.C. Reddy, Syn. Commun., 2010, 40, 39.