Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 2
A Catalyst Free Simple and Efficient One Pot Syntheis of N-benzyloxazolidinone Derivatives
Mahesh Kumar A1,2, Rapolu Venkateshwarlu1, Shambhu Nath Singh1, Chandra Sekhar VN1, Akula Raghunadh1,2*, Venkateswara Rao B3 and Vidavalur Siddaiah22Department of Organic Chemistry and FDW, Andhra University, Visakhapatnam, India
3Department of Engineering Chemistry, Andhra University, Visakhapatnam, India
Akula Raghunadh, Department of Engineering Chemistry, Andhra University, Visakhapatnam, India, Email: raghunadha@drreddys.com
Received: 01-Mar-2024, Manuscript No. Dpc-24-128657; Editor assigned: 06-Mar-2024, Pre QC No. Dpc-24-128657 (PQ); Reviewed: 20-Mar-2024, QC No. Dpc-24-128657; Revised: 22-Mar-2024, Manuscript No. Dpc-24-128657 (R); Published: 19-Apr-2024, DOI: 10.4172/0975-413X.16.2. 243-248
Abstract
An efficient and catalyst free one pot synthesis of N-benzyloxazolidinone derivatives is developed by reaction of N-benzyl-β-amino alcohol derivatives and N, N-Carbonyldiimidazole (CDI) in DMSO. A range of novel N-benzyloxazolidinone derivatives were synthesized by using this one pot methodology with good to excellent yields.
Keywords
Oxazolidinones; N-benzyl-β-amino alcohol; N,N-carbonyldiimidazole; Heterocycles
Introduction
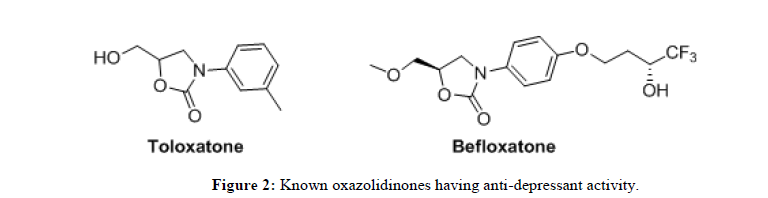
The effectiveness of latest generation of antibiotics is gradually decreasing towards the treatment of numerous infectious diseases due to the newly emerging resistance mechanism developed inside the microbial. This led to the extensive research and there by discovery of new antibiotics for the treatment of various diseases. N-Aryloxazolidinones and the corresponding derivatives are known to be effective against Vancomycin-Resistant Enterococii (VRE) and Methicillin-Resistant Staphylococcus Aureus (MRSA) [1]. The oxazolidinone derivatives, linezolid and posizolid (Figure 1) are found to be active against bacterial infection. Interestingly oxazolidinones also have shown pharmacological activity as antidepressant and antisychosis [2]. Toloxatone and befloxatone (Figure 2) are used for the treatment of depression as a reverse inhibitor of MAO-O [3]. Some of the oxazolidinone derivatives are also employed as key structural fragments in biologically active materials for pharmaceutical use [4] and also as auxiliaries in useful synthetic conversations [5].
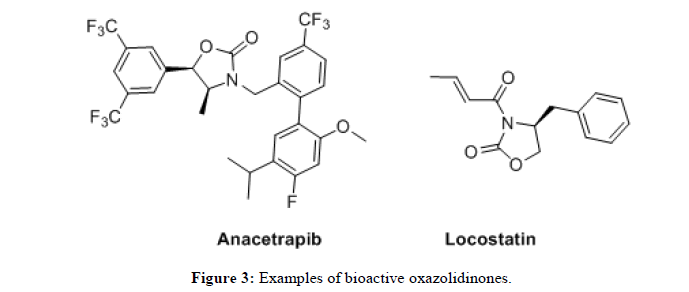
Recently, Anacetrapib which contains an oxazolidine core has been identified as ETP inhibitor (Figure 3) and is currently being studied in clinical trials [6]. Locostatin, an oxazolidinone derivative is found to be useful in controlling inflammation, sepsis and autoimmune diseases [7].
Materials and Methods
The most common synthetic routes reported so far for the synthesis of N-benzyloxazolidinones includes N-benzylayion of oxazolidinones [8], carboxylation of N-benzyl-β-amino alcohols [9]. The methods reported so far either involves use of metal catalyst or use of carbon dioxide in the presence of catalyst.
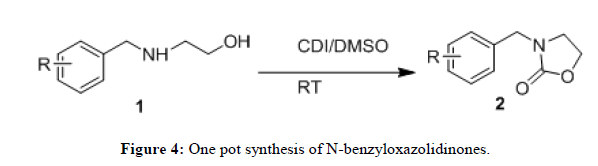
N,N-Carbonyldiimidazole (CDI) is a widely used reagent in organic synthesis. It is frequently used as a replacement for the highly toxic phosgene in reactions with alcohols and amines [10,11] and the by-product imidazole formed after the reaction can be easily removed by dilute acid wash. Based on this, we tried to use CDI as a reagent in our synthesis of N-benzyloxazolidinones and successfully developed a catalyst free approach for the synthesis of N-benzyloxazolidinones (2) from N-benzyl-β-amino alcohol derivatives (1) using N,N-Carbonyldiimidazole (CDI) in DMSO (Figure 4).
Results and Discussion
The N-benzyl-β-amino alcohol derivatives required for the synthesis were prepared from benzaldehyde derivatives and ethanolamine following the same procedure reported by Xiong-Jie and his team. Initially, the reaction was performed by stirring beta amino alcohol 1a and CDI in DCM at room temperature for 15 hrs. To our delight N-benzyloxazolidinone 2a was obtained in 30% yield along with the recovery of the unreacted intermediate N-benzyl-β-amino alcohol (entry 1, Table 1). Attempts were made to increase the product yield by extending the reaction time or temperature (entry 2 and 3, Table 1). But these attempts did not show any significant increase in the product yield. Other solvents like THF, DMSO and DMF (entry 4, 5 and 6, Table 1) were also screened for the reaction. Among the solvents, DMSO was found to be optimum for the reaction and the yield remarkably improved to 94%. Only 25% of the product formation was observed in THF however considerable amount of product (70%) was obtained by using the DMF solvent. Further attempts were made to improve the yield in DMSO by increasing the reaction temperature (entry 7, Table 1) however did not get any positive result. Overall, the reaction conditions presented for the entry 5 in Table 1 found to be superior to other screening experiments.
| Entrya | Solvent | Temp (°C) | Time (h) | % Yieldb |
|---|---|---|---|---|
| 1 | DCM | 30 | 15 | 30 |
| 2 | DCM | 30 | 24 | 32 |
| 3 | DCM | 40 | 15 | 28 |
| 4 | THF | 30 | 15 | 25 |
| 5 | DMSO | 30 | 12 | 94 |
| 6 | DMF | 30 | 15 | 70 |
| 7 | DMSO | 45 | 15 | 40 |
Note: aReactions were carried out using 1a (1.0 mmol) and CDI (1.5 mmol) in a solvent at room temperature under nitrogen; bIsolated yield.
Table 1: Effect of reaction conditions on the conversion of the beta amino alcohol to oxazolidinone derivative.
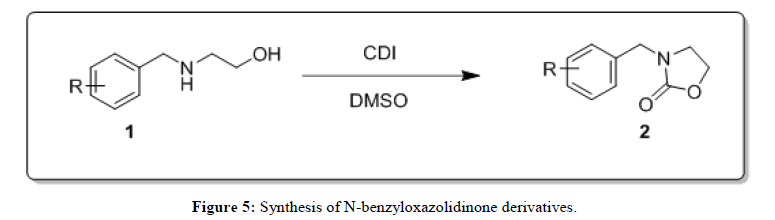
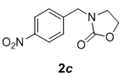
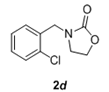
The scope and generality of the reaction was further tested by performing the reactions using variety of N-benzyl-β-amino alcohol derivatives (1). Substituents such as Cl, Br, F, NO2 on the benzene ring (entries 1-6, Table 2) were well tolerated. The reaction proceeded well in all these cases affording desired N-benzylloxazolidinones 2 in good to excellent yields. All the N-benzyloxazolidinone derivatives (2a-h) synthesized were characterized by their 1 H and 13C NMR and HRMS data and further confirmed by comparing with the data reported for these compounds in literature (Figure 5 and Table 3).
| S. NO | N-benzyl-β-amino alcohol derivatives | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 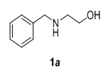 |
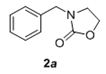 |
2 | 96 |
| 2 | 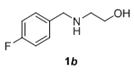 |
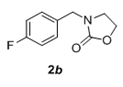 |
2 | 94 |
| 3 | 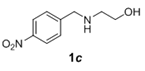 |
 |
3 | 89 |
| 4 | 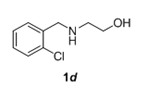 |
 |
3 | 90 |
| 5 | 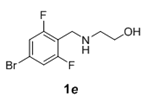 |
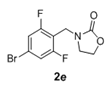 |
2.5 | 92 |
| 6 | 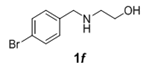 |
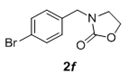 |
2.0 | 90 |
| 7 | 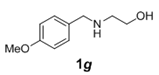 |
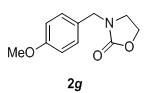 |
3.0 | 88 |
| 8 | 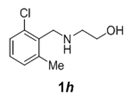 |
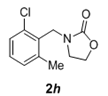 |
2.5 | 90 |
Note: aReactions were carried out using N-benzyl beta amino alcohol (1) (1.0 mmol) and CDI (1.5 mmol) in DMSO at room temperature under nitrogen; bIsolated yield.
Table 2: Synthesis of N-benzyloxazolidinone derivatives 2 via one pot reaction of 1 and CDIa.
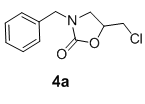
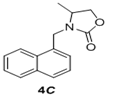
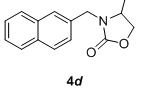
We have also extended this methodology for the preparation of functionalized oxazolidinone derivatives (4a-d, Table 3). The methodology worked well for the synthesis of these derivatives.
| S. No | N-benzyl-β-amino alcohol derivatives | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 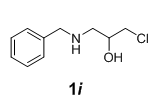 |
 |
2.0 | 96 |
| 2 | 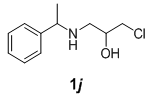 |
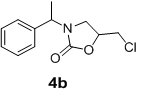 |
2.0 | 94 |
| 3 | 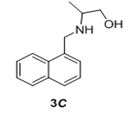 |
 |
3.0 | 89 |
| 4 | 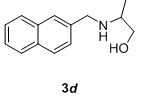 |
 |
3.0 | 90 |
Note: aReactions were carried out using N-benzyl beta amino alcohol (1) (1.0 mmol) and CDI (1.5 mmol) in DMSO at room temperature under nitrogen; bIsolated yield.
Table 3: Synthesis of functionalized oxazolidinone derivatives 4a-d via one pot reaction.
Experimental
General method for the preparation of β-aminoalcohol (1a-h): Ethanolamine (1.0 mmol) was added to a stirring solution of aromatic aldehyde (1 mmol) dissolved in methanol (5 mL) and stirred at 25°C-30°C for 1 h. After 1h, sodium borohydride (0.5 mmol) was added and stirred at 25°C- 30°C for 2 h under nitrogen atmosphere. After the completion of reaction, quenched the reaction mass with water, extracted the product with ethyl acetate and washed with water. Dried the organic layer with sodium sulphate and distilled under vacuum to get the required β-aminoalcohol. 3-benzyloxazolidin-2-one (2a): 1H NMR (400 MHz, CDCl3):δ7.37- 7.26 (m, 5H), 4.42 (s, 2H), 4.31- 4.27(m, 2H), 3.43-3.39(m, 2H); 13CNMR (100 MHz, CDCl3): δ158.53, 135.76, 128.83(2C), 128.16(2C), 127.98, 61.76, 48.42, 43.95; HRMS: m/z [M+H] calculated for C10H12NO2: 178.0868; found: 178.0865.
General method for the preparation of oxazolidinones derivatives (2a-2h) and (4a-d): β-Aminoalcohol (1.0 mmol) was dissolved in DMSO (2 mL) and added carbonyldiimidazole (1.5mmol) under nitrogen atmosphere and stirred at 23°C-30°C for 2 h-3 h. After the completion of reaction, reaction mass was diluted with water and extracted the product with ethyl acetate. Washed the ethyl acetate layer with dilute HCl solution followed by with water. Dried the organic layer with sodium sulphate and distilled under vacuum to get the pure product.
4-methyl-3-(naphthalen-1-ylmethyl)oxazolidin-2-one (4c): 1H NMR (400 MHz, CDCl3): δ 8.10-8.08 (d, J=7.9Hz, 1H), 7.97-7.95 (m, 1H), 7.91- 7.89 (d, J=7 Hz, 1H), 7.59-7.51 (m, 2H), 7.49-7.45 (m, 2H), 4.85-4.82 (d, J=14.9 Hz, 1H), 4.74-4.71 (d, J=14.9Hz , 1H), 4.58-4.53 (m, 1H), 3.45- 3.41 (t, 1H), 2.91-2.87 (t, 1H), 1.20-1.19 (d, J=6.1Hz, 3H); 13C NMR (100MHz, CDCl3): 157.59, 133.94, 131.49, 129.13, 128.94, 127.28, 127.03, 126.51, 125.88, 123.72, 70.31, 50.59, 45.93, 20.81; HRMS: m/z [M+H] calculated for C15H15NO2: 242.118; found: 242.1188.
Conclusion
In conclusion, we have reported a simple and efficient one pot synthesis of N-benzyloxazolidinones from N-benzyl-β- amino alcohols and CDI in DMSO in good to excellent yields. The methodology does not require any use of base, catalyst or hazardous solvents.
Acknowledgment
The authors would like thank the management of Dr. Reddy’s laboratories limited for providing support to carry out this work. We also thank the analytical department of Dr. Reddy’s laboratories for providing timely support.
References
- Crich D. J Org Chem. 2011; 76(22): p. 9193-9209.
[Crossref] [Google Scholar] [PubMed]
- Christensen HM, Oscarson S, Jensen HH. Carbohydr Res. 2015; 408: p. 51-95.
[Crossref] [Google Scholar] [PubMed]
- Van Delft FL, Timmers CM, Van der Marel GA, et al. Synthesis. 1997; 1997(04): p. 450-454.
- Park CS, Kim MS, Sim TB, et al. J Org Chem. 2003; 68(1): p. 43-49.
[Crossref] [Google Scholar] [PubMed]
- Ella-Menye JR, Wang G. Tetrahedron. 2007; 63(40): p. 10034-10041.
- Mancuso R, Maner A, Ziccarelli I, et al. Molecules. 2016; 21(7): p. 897.
[Crossref] [Google Scholar] [PubMed]
- Buur A, Bundgaard H. Int J Pharm. 1988; 46(1-2): p. 159-167.
- Lindsay KB, Ferrando F, Christensen KL, et al. J Org Chem. 2007; 72(11): p. 4181-4188.
[Crossref] [Google Scholar] [PubMed]
- Sonzini P, Damiano C, Intrieri D, et al. Adv Synth Catal. 2020; 362(14): p. 2961-2969.
- Pulla S, Felton CM, Ramidi P, et al. J CO2 Util. 2013; 2: p. 49-57.
- Peilleron L, Retailleau P, Cariou K. Adv Synth Catal. 2019; 361(22): p. 5160-5169.