Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
6ꞌ,7ꞌ-Dihydroxybergamottin Promotes Collagen Synthesis by Activating the Smad Signaling Pathway in Human Dermal Fibroblasts
Ji-Seon Hyun1, Sang Suk Kim2, Kyung Jin Park2, Hyun Ju An2, Young Hun Choi2, Nam Ho Lee1 and Chang-Gu Hyun1*
1Department of Chemistry and Cosmetics, Jeju National University, Jeju-63243, Korea
2Citrus Research Institute, National Institute of Horticulture and Herbal Science, RDA, Seogwipo-63607, Korea
- *Corresponding Author:
- Chang-Gu Hyun
Department of Chemistry and Cosmetics
Jeju National University
Jeju-63243, Korea
Abstract
Skin wrinkling appears to be principally related to decrease levels of type I collagen, elastin, and hyaluronic acid, the principal components of the dermal layer of skin. The cause of wrinkling and the appearance of the skin resulting from decreased elasticity and skin sagging and pigmentation, is generally associated with decreased collagen and increased collagenase and Matrix Metalloproteinase (MMP) activities. In this study, we investigated the possibility that 6ꞌ,7ꞌ-Dihydroxybergamottin (DHB) may be an effective anti-wrinkle agent. DHB is a natural component of furanocoumarin which is frequently observed in citrus fruits. Initially, up to 200 μM DHB was shown to be non-toxic to human dermal fibroblasts. Then, a human type I pro-collagen production assay was performed in human dermal fibroblast cells treated with 50, 100, and 200 μM DHB. The results showed that DHB significantly increased type I pro-collagen production. In addition, DHB strongly inhibited MMP-1 production in a concentration-dependent manner. DHB was also found to induce the phosphorylation of Smad2, an important transcription factor involved in the production of type I procollagen. These results suggest that DHB may be a potential anti-wrinkle agent for topical application in the cosmetic industry.
Keywords
Anti-aging, 6,7-dihydroxy bergamottin, Type 1 procollagen, MMP-1, Smad signaling pathway
Introduction
Skin aging is a complex process that produces functional and structural alterations to the skin and causes various physiological changes with advancing age. There are two distinct types of skin aging [1,2]. Intrinsic aging is induced by internal factors such as cellular metabolism and hormone and metabolic processes, whereas extrinsic aging is primarily caused by chronic light exposure, pollution, ionizing radiation, chemicals, and toxins. Wrinkle formation is a representative feature of skin aging and is characterized by reduced levels of extracellular matrix components, such as collagen and elastin, that are produced by fibroblasts in the dermis and a decrease in skin elasticity and tension [3,4].
Matrix Metalloproteinase (MMPs), which are zinc-dependent proteins, degrade all types of extracellular matrix and damage connective tissues [5,6]. Depending on the substrates, MMPs can be categorized into collagenases, gelatinases and stromelysins. MMP-1 and -13 degrade native collagens as collagenases, whereas MMP-2 and -9 cleave the basement membrane as gelatinases. On the other hand, MMP-3 and -10 confer broader substrate specificity than collagenases and gelatinases as stromelysins [7]. Among these enzymes, active MMP-1 and interstitial collagenase in dermal fibroblasts are responsible for the degradation of collagen fibers. Thus, increased expression/activity of MMP-1 and reduced production of type I collagen by dermal fibroblasts, are prominent features of aged human skin [8]. For this reason, natural compounds possessing the ability to promote collagen synthesis or inhibit the major MMP enzymes are potentially useful as anti-wrinkle cosmetic agents.
Type I collagen accounts for 70-90% of the total 29 types found in human dermis [9,10]. Alterations in the structure of type I collagen have been considered to be a primary cause of skin aging and wrinkle formation [11,12]. Metabolic control of type I collagen may, therefore, be useful for a diversity of cosmetic materials [13].
Collagen synthesis is regulated both transcriptionally and post-transnationally. The Smad pathway is involved in the activation of type I collagen gene expression. The Smads are a series of proteins that perform functions downstream from the serine/threonine kinase receptors of the Transforming Growth Factor (TGF) family, thereby transducing signals to the nucleus [14]. Several natural products including Panax ginseng, Camellia japonica oil and Pyropia yezoensis peptide, have been suggested recently as possible collagen upregulating and anti-wrinkle agents, function through the Smad pathways in human dermal fibroblasts [15-17].
6ꞌ,7ꞌ-Dihydroxybergamottin (DHB) is a furanocoumarin that is abundant in citrus fruits. It is a clinically active compound capable of inhibiting cytochrome P450 3A4. Recently, our lab reported the anti-inflammatory effect of DHB on lipopolysaccharide-stimulated RAW 264.7 macrophage cells [18]. However, no other dermatologic effects have been reported.
In this study, we demonstrated that DHB significantly increased type I pro-collagen production without being cytotoxic to human dermal fibroblast. In addition, DHB strongly inhibited MMP-1 production in a concentration-dependent manner. More importantly, for the first time, we elucidated the molecular mechanisms underlying the inhibitory effect of DHB by showing that TNF-α-induced type I pro-collagen production is mediated by phosphorylation of Smad 2 and this effect is inhibited by DHB. These data suggest that DHB has anti-wrinkle activity in TNF-α- stimulated human dermal fibroblasts.
Materials and Methods
Materials
DHB and the Smad2 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), the procollagen type I C-peptide (PIP) enzyme immunoassay kit from Takara Bio (Kusatsu, Japan), and human pro-MMP-1, Transforming Growth Factor (TGF)-β1 from R & D Systems (Minneapolis, MN, USA), Tumor Necrosis Factor (TNF)-α, radioimmuno-precipitation assay buffer were purchased from Sigma- Aldrich (St. Louis, MO, USA), Antibodies against phosphor Smad2, β-actin and horseradish peroxidase-conjugated IgG were purchased from Cell Signaling Technology (Danvers, MA, USA), Bicinchoninic Acid (BCA) protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA), polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA).
Cell lines and cell culture
Human dermal fibroblast cells were kindly donated by LG HG & CM Research Institute (Daejeon, Korea). Cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 1% penicillin/streptomycin (50 unit/ml and 50 μg/ml, respectively) and 30% F-12 nutrient mixture at 37°C in a 5% CO2 incubator.
Cell viability
Cell viability was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (5 × 104/ml) were plated into 24-well plates and incubated for 24 h. The cells were then treated with 0, 50, 100 or 200 μM DHB using serum-free medium, followed by incubation for 24 h at 37°C. The MTT solution (0.4 mg/ml) was added to the medium for 4 h. Supernatants were then removed and the formazan formed was dissolved in Dimethyl Sulphoxide (DMSO). Absorbance was measured at 540 nm using a microplate reader. Cytotoxicity was evaluated by comparing the absorbance of DHB-treated and untreated samples.
Measurement of type 1 pro-collagen levels
The amount of type I pro-collagen in the cells was measured by using a PIP enzyme immunoassay kit in accordance with the manufacturer’s instructions. Cells (5 × 104/ml) were cultured in 24-well plates and incubated for 24 h. DHB and/or TGF-β (10 ng/ml) were then added to each well and incubated for an additional 24 h. The supernatants were collected and the absorbance was measured at 450 nm using a microplate reader.
Measurement of MMP-1 production
MMP-1 production in human dermal fibroblasts was measured using a human pro-MMP-1 immunoassay kit in accordance with the manufacturer’s instructions. Cells (5 × 104/ml) were plated into 24-well plates and incubated for 24 h. Then, DHB and/or TNF-α (10 ng/ml) were added to each well and incubated for 24 h. The supernatants were collected the absorbance was measured at 450 nm using a microplate reader.
Western blot analysis
Cells (1.0 × 106/well) were cultured in 100 mm petri dishes at 37°C in a 5% CO2 incubator. Cells were treated with various concentrations of DHB and/or TGF-β (10 ng/ml) in serum-free medium. Cells were washed twice with phosphate-buffered saline, lysed with radioimmunoprecipitation assay buffer, and centrifuged at 15,000 rpm for 40 min. Protein concentrations were determined using bovine serum albumin as a standard with a bicinchoninic acid protein assay kit. Proteins were separated on a polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and blocked for 3 h at room temperature using blocking buffer (5% skim milk in Tween 20/Tris-Buffered Saline (TTBS)). The membranes were washed six times with TTBS and the diluted primary antibody (1:1000) was added at 4°C overnight. After completion of the primary antibody reaction, cells were washed six times with TTBS, reacted with the diluted secondary antibody (1:3000) at room temperature for 2 h and then washed six more times with TTBS. Proteins were detected using enhanced chemilumescence.
Statistical analysis
All data were obtained from three independent experiments. Data are expressed as means ± standard deviation. Statistical significance was verified by Student's t-test.
Results and Discussion
DHB is a furanocoumarin derivative found in various citrus fruits, particularly grapefruit. It was reported previously that DHB has physiological activities such as inhibiting P450 enzymes and anti-inflammation. Generally, skin aging has been reported to involve the inhibition of collagen biosynthesis by stress and various external environmental factors. In addition, wrinkle formation is induced by increasing the expression of MMPs that interfere with collagen deposition. Therefore, this study was conducted to determine whether DHB was effective and suitable as a natural anti-wrinkle cosmetic ingredient.
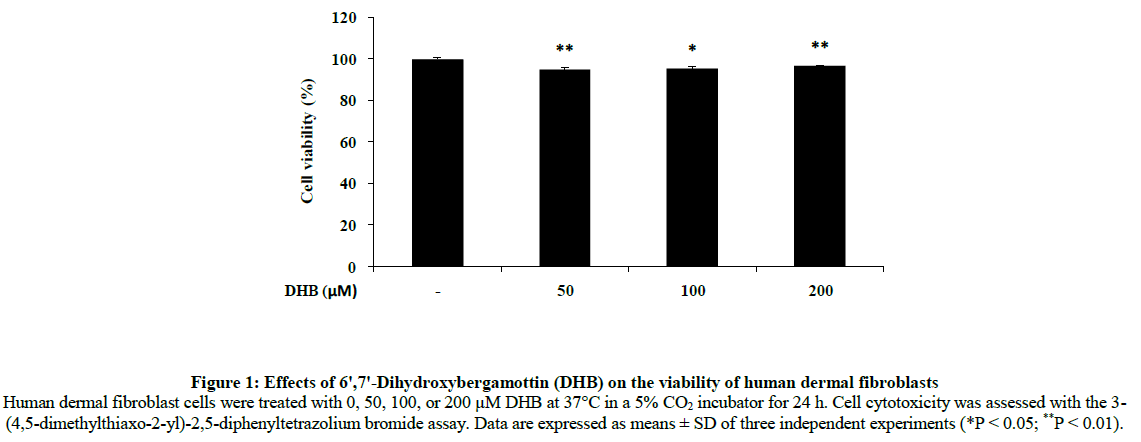
Initially, human dermal fibroblasts cells were treated with 50, 100 or 200 μM DHB for 24 h to determine the maximum concentration appropriate for future experiments. As shown in Figure 1, there was no significant difference in the viability between control and DHB-treated cells at any concentration tested.
Figure 1: Effects of 6ꞌ,7ꞌ-Dihydroxybergamottin (DHB) on the viability of human dermal fibroblasts Human dermal fibroblast cells were treated with 0, 50, 100, or 200 μM DHB at 37°C in a 5% CO2 incubator for 24 h. Cell cytotoxicity was assessed with the 3- (4,5-dimethylthiaxo-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are expressed as means ± SD of three independent experiments (*P < 0.05; **P < 0.01).
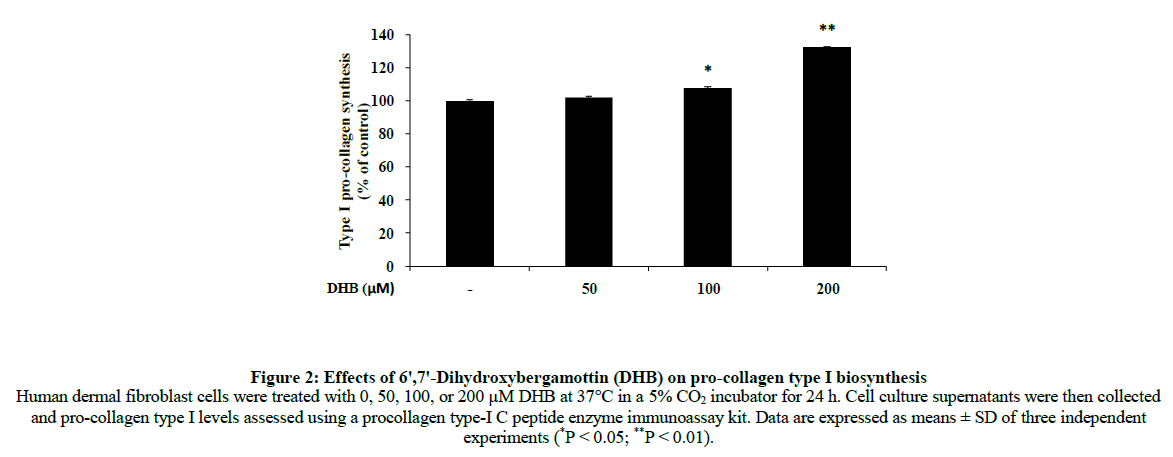
Collagen is a major structural protein that maintains the elasticity of skin and is distributed widely in the dermal layer [19]. Because a reduction of collagen is closely related to the cause of wrinkle formation, many studies have been conducted to delay and prevent this change. To investigate whether DHB enhanced the production of type I procollagen in human dermal fibroblasts, we treated cells with 50, 100 and 200 μM DHB and monitored levels of type I procollagen by an enzyme-linked immunosorbent assay. The procollagen content of cells was significantly increased by DHB in a dose-dependent manner (Figure 2).
Figure 2: Effects of 6ꞌ,7ꞌ-Dihydroxybergamottin (DHB) on pro-collagen type I biosynthesis Human dermal fibroblast cells were treated with 0, 50, 100, or 200 μM DHB at 37°C in a 5% CO2 incubator for 24 h. Cell culture supernatants were then collected and pro-collagen type I levels assessed using a procollagen type-I C peptide enzyme immunoassay kit. Data are expressed as means ± SD of three independent experiments (*P < 0.05; **P < 0.01).
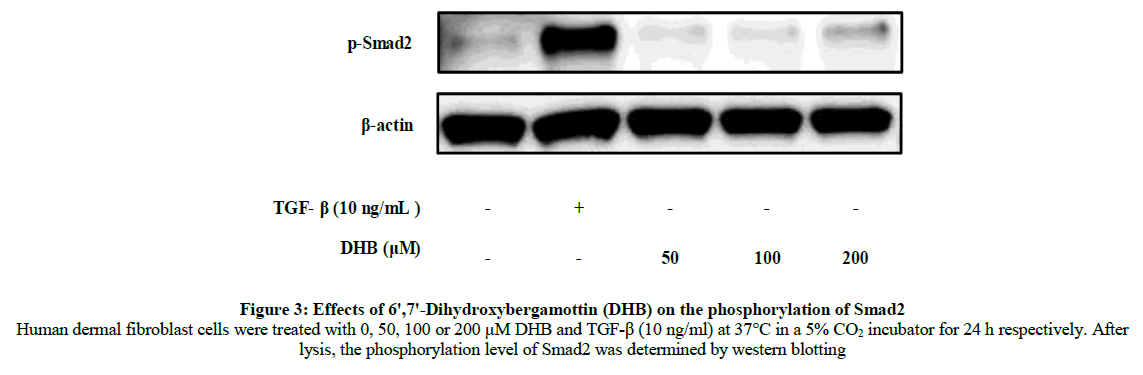
We next investigated the mechanism by which DHB promoted type I procollagen production in human dermal fibroblasts. Because Smad pathways are important in transducing extracellular signals to effect the intracellular response, we determined whether this pathway was involved in DHB-induced type I procollagen expression. To see whether DHB increased the expression of active Smads, we monitored the phosphorylation level of Smad2 after treatment of human dermal fibroblasts with DHB. DHB significantly increased Smad2 phosphorylation at a concentration of 200 μM (Figure 3). This finding shows that DHB strongly enhances collagen synthesis by markedly increasing the phosphorylation of Smad2. These results indicate that DHB effectively activates collagen biosynthesis through up-regulation of Samd2 and thus could be useful as a new anti-wrinkle agent.
Figure 3: Effects of 6ꞌ,7ꞌ-Dihydroxybergamottin (DHB) on the phosphorylation of Smad2 Human dermal fibroblast cells were treated with 0, 50, 100 or 200 μM DHB and TGF-β (10 ng/ml) at 37°C in a 5% CO2 incubator for 24 h respectively. After lysis, the phosphorylation level of Smad2 was determined by western blotting
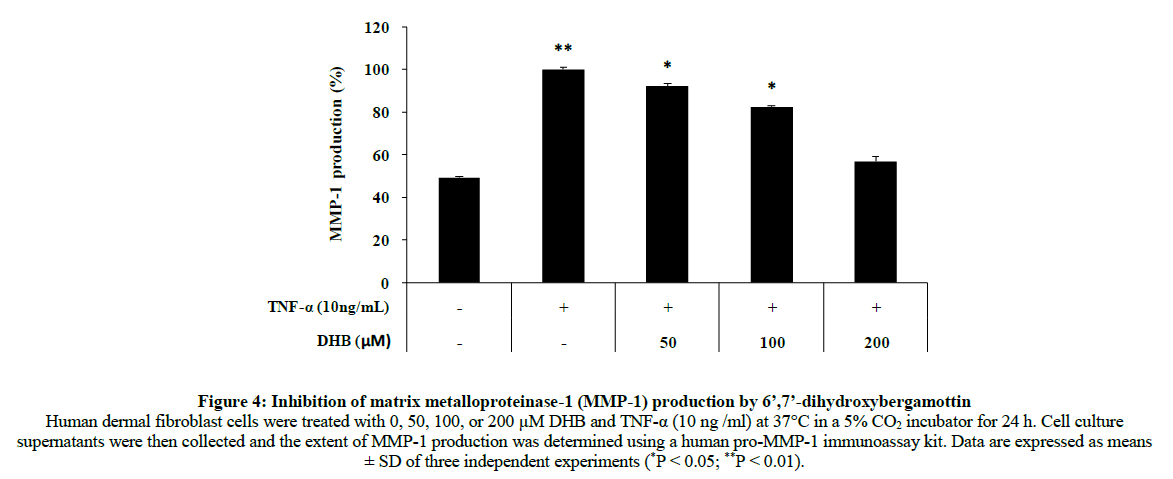
Collagen is mainly responsible for dermal strength and resiliency. External irritation activates MMPs that attack and degrade collagen, resulting in skin aging. Ultimately, collagen degradation weakens the structural integrity of fibers during the process of skin aging. Thus, inhibiting collagenase could be a useful strategy for preventing skin aging. We examined the effects of DHB on MMP-1 production using a pro-MMP-1 immunoassay. The MMP-1 level increased 50.7% compared with the control group after treatment of human dermal fibroblasts with 10 ng/ml TNF-α. Treatment with DHB significantly decreased 43.2% (Figure 4). These results indicated that DHB dose-dependently inhibited TNF-α- induced MMP-1 production.
Figure 4: Inhibition of matrix metalloproteinase-1 (MMP-1) production by 6’,7’-dihydroxybergamottin Human dermal fibroblast cells were treated with 0, 50, 100, or 200 μM DHB and TNF-α (10 ng /ml) at 37°C in a 5% CO2 incubator for 24 h. Cell culture supernatants were then collected and the extent of MMP-1 production was determined using a human pro-MMP-1 immunoassay kit. Data are expressed as means ± SD of three independent experiments (*P < 0.05; **P < 0.01).
Based on these results, we suggest that DHB can be considered as a potential anti-wrinkle candidate for topical application. However, although anti-wrinkle effects of DHB were identified, the precise mechanism of action was not determined. Thus, possible inhibitory mechanisms toward MMP-1 activity remain to be evaluated. To date, several important common pathways have been identified. One such pathway involves signaling through mitogen-activated protein kinases. These enzymes control the expression of MMPs that contain an AP-1 regulatory element, and several factors such as MAP kinases regulate AP-1 expression [20-22].
Conclusion
In recent years, many natural product-derived agents claiming anti-aging activity have been used as ingredients in skin care products. In particular, inhibitors of collagenase expression and stimulators of collagen production are considered to be effective against connective tissue damage associated with skin aging. In the present study, DHB exhibited a strong potential for use in anti-aging cosmetic formulations because it affected pathways associated with skin aging in human dermal fibroblasts by reducing MMP-1 production and increasing procollagen secretion. In addition, DHB increased the phosphorylation level of Smad2 leading to an increase in collagen synthesis. However, the mechanism by which DHB inhibits MMP-1 production in human dermal fibroblast cells remains unclear. Investigations of the exact signaling pathways, such as MAPKs, and additional in vivo experiments, are needed to evaluate the use of DHB as a natural anti-wrinkle agent.
Acknowledgement
This work was supported by the Project_"PoINT" of Jeju National University in 2016.
References
- N. Azmi, P Hashim, D.M. Hashim, N. Halimoon, N.M.N. Majid, Asian Pac. J. Trop. Biomed., 2014, 4, S348-S352.
- H.N. Jung, E.H. Lee, T.H. Lee, M.H. Cho, Int. J. Mol. Sci., 2016, 17, 1449.
- M.K. Kim, C.Y. Bang, G.J. Yun, H.Y. Kim, Y.P. Jang, S.Y. Choung, BMC Complementary and Alternative Medicine., 2016, 16, 116.
- Z.W. Sun, S.Y. Park, E.S. Hwang, B. Park, S.A. Seo, J.G. Cho, M.Y. Zhang, T.H. Yi, Phytomedicine., 2016, 23, 1273-1284.
- P. Pavida, M. Jitlada, P. Ornicha, K. Mayumi, O. Mamitaro, Int. J. Mol. Sci., 2016, 17, 868.
- J.L. Gustavo, G.K. Ana, E.B. María, H. Karin, Journal of Dermatological Science., 2017, 85, 124-130.
- J.W. Kim, M.B. Kim, J.G. Yun, J.K. Hwang, J. Microbiol. Biotechnol., 2017, 27, 242-250.
- C.S. Lee, I.H. Bae, J.W. Han, G.Y. Choi, K.H. Hwang, D.H. Kim, M.H. Yeom, Y.H. Park, M.Y. Park, Exp. Dermatol., 2014, 23, 819-824.
- E.S. Hwang, S.Y. Park, C.S. Yin, H.T. Kim, Y.M. Kim, T.H. Yi, J. Ginseng Res., 2017, 41, 69-77.
- B.G. Ha, M.A. Park, C.M. Lee, Y.C. Kim, Toxicol. Res., 2015, 31, 363-369.
- Y.F. Hong, H.Y. Lee, B.J. Jung, S.J. Jang, D.K. Chung, H.G. Kim, Mol. Immunol., 2015, 67, 248-255.
- D.H. Kim, T.S. Park, J.H. Son, J. Appl. Biol. Chem., 2016, 59, 317-322.
- J.H. Park, S.R. Kim, H.J. An, W.J. Kim, M. Choe, J.A. Han, Mol. Cell Biochem., 2012, 368, 61-67.
- P. Trupta, H. Tianyuan, Q. Zhaoping, L. Ting, J.F. Gary, Y. Yan, J.V. John, Q. Taihao, J. Dermatol. Sci., 2016, 83, 70-83.
- E.S. Jung, J.S. Lee, J.H. Baek, K.S. Jung, J.Y. Lee, S.R. Huh, S.B. Kim, J.S. Koh, D.H. Park, J. Ethnopharmacol., 2007, 112, 127-131.
- J.S. Lee, E.S. Jung, J.Y. Lee, S.R. Huh, J.E. Kim, M.J. Park, J.W. So, Y.G. Ham, K.S. Jung, C.G. Hyun, Y.S. Kim, D.H. Park, J. Ethnopharmacol., 2007, 109, 29-34.
- C.R. Kim, Y.M. Kim, M.K. Lee, I.H. Kim, Y.H. Choi, T.J. Nam, Int. J. Mol. Med., 2017, 39, 31-38.
- M.J. Kim, J.S. Hyun, K.N. Kim, S.S. Kim, K.J. Park, H.J. An, Y.H. Choi, N.H. Lee, C.G. Hyun, Latin American Journal of Pharmacy, 2017, 36(2), 340-345.
- J.S. Bae, M.R. Han, H.S. Shin, M.K. Kim, C.Y. Shin, D.H. Lee, J.H. Chung, J. Ethnopharmacol., 2017, 195, 334-342.
- H.M. Chiang, C.W. Che, T.Y. Lin, Y.H. Kuo, Food Chem. Toxicol., 2014, 72, 154-161.
- B.C. Yu, D.S. Lee, S.M. Bae, W.K. Jung, J.H. Chun, S.H. Urm, D.Y. Lee, S.J. Heo, S.G. Park, S.K. Seo, J.W. Yang, J.S. Choi, W.S. Park, I.W. Choi, Life Sci., 2013, 92, 282-288.
- H.J. Lee, E.S. Hwang, B. Park, M.Y. Zhang, Z.W. Sun, D.G. Lee, S.Y. Park, T.H. Yi, Phytother. Res., 2016, 30, 1519-1526.