Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
2,2'-Bipyridine: Efficient Ligand for the Copper Acetate Catalyzed ChanÃÆâÃâââ¬Ãâââ¬ÅLam Coupling Reaction under Ultrasonic Irradiation
Subbareddy M*
S.B.V.R. Degree College, Deparment of Chemistry, Badvel-516227, Andhra Pradesh, India
- *Corresponding Author:
- Subbareddy M
S.B.V.R. Degree College
Deparment of Chemistry
Badvel-516227, Andhra Pradesh, India
Abstract
An efficient and convenient approach for the synthesis of diaryl ethers via the one-pot synthesis of and aryl boronic acids and phenols in the presence of copper acetate and 2,2'-bipyridine under ultrasonic irradiation. This procedure has high conversions with good product yields, less reaction time, gainful cost, air stable, easy purification, and environmentally benign reaction conditions. All synthesized compounds were characterized by spectral techniques, like Proton Nuclear Magnetic Resonance (1H-NMR), Carbon-13 Nuclear Magnetic Resonance (13C-NMR) and Mass Spectrometry (MS).
Keywords
2, 2'-bipyridine, Aryl boronic acids, Phenols, Ultrasonic irradiation.
Introduction
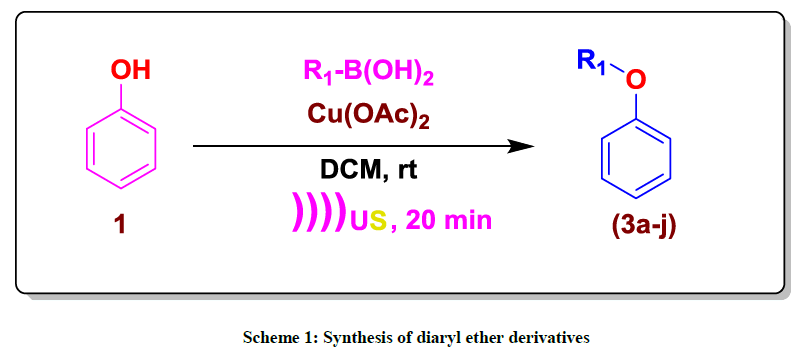
Most of the diaryl ethers (Scheme 1) are biologically active; therefore the enhancement of efficient methods for their preparation is an keenly considered area [1]. Diaryl ethers are prepared by Hartwig-Buchwald coupling [2], Ullmann coupling [3] and Chan–Lam coupling reactions [4,5]. The copper (II) acetate promoted coupling of arylboronic acids and phenols under mild conditions was reported independently by Evans [4,5] and Chan [6]. Several copper salts were tested and copper (II) acetate proved to be most efficient. However, some other promoters including Cu(OTf)2 and Cu2O, CuF were also described [7-9]. Their methods made use of stoichiometric amounts of copper catalyst and boronic acids as the aryl donors. In the presence of a base, the coupling could be performed at room temperature. These reactions were subsequently shown to work with a large number of nucleophiles and tolerated a variety of substrates, construction the process one of the most efficient ways for C–O coupling [10]. Some modifications of the Chan–Lam reaction have been reported, expanding its capacity and it has since been used to synthesise several biologically active compounds [10,11]. In most cases the promoter is used in equimolar quantity and also oxygen from air is necessary. However, these methods suffer from drawbacks, such as poor yields, more time consuming reaction, restricted subsrate scoped. Thus, it is still desired to general and efficient method. We became interested in developing efficient method via Chan–Lam coupling of phenol with aryl boronic acids under ultrasound irradiation. Here in, we disclose our recent efforts for Chan–Lam coupling of phenol with aryl boronic acids catalyzed by the combination of Cu(OAc)2 and 2,2'-bipyridine. The reaction is usually finished 20 min with good to excellent isolated yields [12,13].
Material and Methods
Experimental
All the chemicals, solvents, and reagents were purchased from Merck (Mumbai, India), Lancaster Chemical (Mumbai, India), Sigma-Aldrich (Hyderabad, India), and SD fine chemicals All used solvents for spectroscopic and other physical studies were reagent grade and were further purified by employing the reported methods. All the NMR spectra were recorded on Bruker 400 MHz and 300 MHz spectrometer operating at 400 MHz and 300 MHz for Proton Nuclear Magnetic Resonance (1H-NMR), and 100 MHz for Carbon-13 Nuclear Magnetic Resonance (13C-NMR). The compounds were dissolved in CDCl3 and Dimethyl Sulfoxide-d6 (DMSO-d6); the chemical shifts were referenced to TMS. Coupling constants were calculated in hertz (Hz).
Genaral procedure for synthesized compounds
Commercially available compound 1a (1 equiv), aryl boronic acids (2a-j) (1.0 equiv), 2,2'-bipyridine (0.2 equiv) were mixed in dichloromethane (5 ml) and 1 mol% amount of catalyst (Cu(OAc)2 was added. The reaction mixture was stirred at rt for 20 min. After completion of reaction (TLC), the reaction mass was filtered through pad of celite, rinsed with dichloromethane, filtrate was concentrated under vacuum. The residue was purified by flash chromatography to afford corresponding desired product 88% yield.
Synhesis of 1-Ethyl-3-phenoxybenzene (3a): Yellowish oil;1H-NMR (CDCl3): 7.33 (dd, 2H, J=7.9 Hz), 7.24 (dd, 1H, J=7.8 Hz), 7.10 (t, 1H, J=7.4 Hz), 7.02 (d, 2H, J=8.5Hz), 6.95 (d, 1H, J=7.6 Hz), 6.89 (s, 1H), 6.82 (d, 1H, J=8.1 Hz), 2.64 (q, 2H, J=7.6 Hz), 1.23 (t, 3H, J=7.6 Hz); 13C-NMR (CDCl3): 157.60, 157.40, 146.60, 129.90, 129.70, 123.25, 123.10, 119.00, 118.70, 116.30, 29.10 and 15.60.
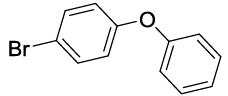
Synhesis of 1-Bromo-4-phenoxybenzene (3b): Yellowish oil; 1H-NMR (CDCl3): 7.45 (d, 2H, J=8.6 Hz), 7.37 (dd, 2H, J=7.8 Hz), 7.15 (t, 1H, J=7.5 Hz,), 7.02 (d, 2H, J=8.0 Hz), 6.91 (d, 2H, J=8.6 Hz); 13C-NMR (CDCl3): 156.90, 156.80, 132.90, 130.10, 123.90, 120.65, 119.25, and 115.80.
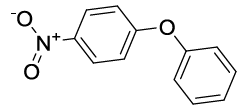
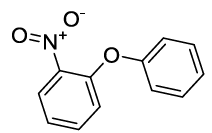
Synhesis of 1-Nitro-4-phenoxybenzene (3c): Yellowish oil; 1H-NMR (CDCl3): 8.22 (d, 2H, J=9.0 Hz), 7.46 (dd, 2H, J=7.5 Hz), 7.28 (t, 1H, J=7.1 Hz), 7.11 (d, 2H, J=7.8Hz), 7.03 (d, 2H, J=8.6 Hz); 13C-NMR (CDCl3): 163.40, 154.80, 142.70, 130.40, 125.98, 125.45, 120.58, and 117.15.
Synhesis of Diphenyl ether (3d): Yellowish oil; 1H-NMR (CDCl3): 7.37 (dd, 4H, J=7.9 Hz), 7.13 (t, 2H, J=7.4 Hz), 7.04 (d, 4H, J=7.7 Hz); 13C-NMR (CDCl3): 157.47, 129.95, 123.43 and 119.10.
Synhesis of 3-Ethoxy-4-phenoxybenzaldehyde (3e): White solid; 1H-NMR(CD Cl3): 9.91 (s, 1H), 7.53 (s, 1H), 7.38 (m, 3H, J=7.9 Hz), 7.18 (t, 1H, J=7.3 Hz), 7.06 (d, 2H, J=7.9 Hz,), 6.98 (d, 1H, J=8.1 Hz), 4.19 (q, 2H, J=6.9 Hz), 1.42 (t, 3H, J=6.9 Hz); 13C-NMR(CDCl3): 191.00, 156.30, 152.30, 150.50, 132.40, 129.85, 125.50, 124.10, 119.20, 118.54, 112.15, 64.74, 14.60.
Synhesis of 1-Nitro-2-phenoxybenzene (3f): Yellow solid; 1H-NMR (CDCl3): 8.21-8.19 (s, 1H), 7.53 (m, 2H), 7.45-7.41 (m, 3H), 7.27-7.26 (d, 1H, J=7.2 Hz), 7.10-7.08 (m, 2H), 7.02-7.0 (m, 2H), 13C-NMR (CDCl3): 163.40, 154.77, 142.71, 130.33, 125.42, 120.54, 117.13.
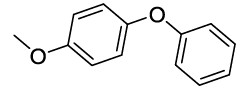
Synhesis of 1-methoxy-4-phenoxybenzene (3g): Yellowish oil; 1H-NMR (CDCl3): 7.23-7.17 (m, 2H), 6.98-6.78 (m, 7H), 3.72 (s, 1H); 13C-NMR (CDCl3): 158.50, 155.90, 150.15, 129.61.
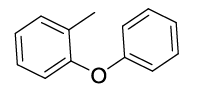
Synhesis of 1-methyl-2-phenoxybenzene (3h): Yellowish oil; 1H-NMR (CDCl3): 7.27-7.13 (m, 3H), 7.12-7.04 (m, 1H), 7.02-6.91 (m, 2H), 6.83-6.81 (m, 3H), 2.16 (s, 1H); 13C-NMR (CDCl3): 157.90, 154.45, 131.40, 130.10, 129.64, 127.12, 124.00, 122.32, 119.80, 117.29 and 16.20.
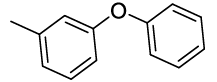
Synhesis of 1-methyl-3-phenoxybenzene (3i): Colourless oil; 1H-NMR (CDCl3): 7.26-7.22 (m, 2H), 7.16-7.10 (m, 1H), 7.03-6.98 (m, 1H), 6.94-6.91 (m, 2H), 6.84 (d, 1H, J=7.2 Hz), 6.75-6.71 (m, 2H), 2.47 (s, 3H); 13C-NMR (CDCl3): 157.40, 157.20, 140.00, 126.69, 129.44, 124.10, 123.10, 120.0, 118.86, 116.0 and 21.39.
Synthesis of 1-Phenoxy-3-(trifluoromethyl) benzene (3J): Colourless oil; 1H-NMR (CDCl3): 7.37-7.28 (m, 4H), 7.26 (d, 1H, J= 7.6 Hz), 7.17-7.07 (m, 2H), 6.96 (d, 2H, J=8.0 Hz); 13C-NMR (CDCl3): 157.90, 156.16, 132.10, 130.04, 124.19, 121.60, 119.60, 119.58, 119.43, and 115.30.
Result and Discussion
The coupling reaction between Phenol and (3-ethylphenyl) boronic acid was selected as a model reaction for optimizing the reaction conditions under ultrasound irradiation. Results are shown in Table 1. In an initial experiment, we exposed that the reaction performed under air thus, improving its operational simplicity. We explored the effect of catalyst on the model reaction (Tables 1 and 2). The yields of the product shown in Table 2, Among them, Cu(OAc)2 and 2, 2'-bipyridine combination is best for this reaction system.
| S. No. | Catalyst | ligand | Temperature (ºC) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | Cu(OTf)2 | 2,2'-bipyridine | rt | 20 | 40 |
| 2 | Cu2O | 2,2'-bipyridine | rt | 20 | 30 |
| 3 | CuF | 2,2'-bipyridine | rt | 20 | 62 |
| 4 | CuI | 2,2'-bipyridine | rt | 20 | 40 |
| 5 | Cu(OAc)2 | 2,2'-bipyridine | rt | 20 | 88 |
a Reaction conditions: Phenol (1.0 eq), (3-ethylphenyl)boronic acid (2a) (1.0 eq), ligand (0.2 eq), catalyst amount (1 mol%), dichloromethane, under ultrasonic irradiation. The reactions were monitored by TLC. b Isolated yields.
Table 1: Optimum conditions for Suzuki coupling of Phenol with and (3-ethylphenyl) boronic acid
| S. No. | Aryl boronic acids (2a-j) | Product (3a-j) | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| 01 | 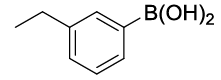 |
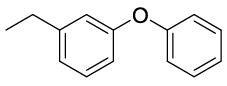 |
20 | 88 |
| 02 | 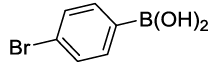 |
 |
20 | 85 |
| 03 | 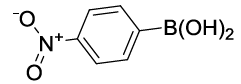 |
 |
25 | 77 |
| 04 | 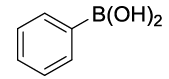 |
 |
20 | 89 |
| 05 | 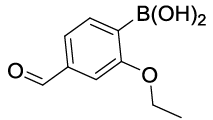 |
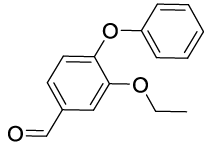 |
30 | 30 |
| 06 | 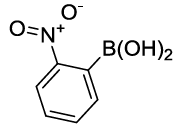 |
 |
25 | 70 |
| 07 | 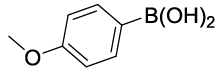 |
 |
20 | 84 |
| 08 | 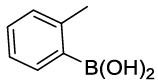 |
 |
20 | 82 |
| 09 | 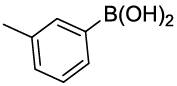 |
 |
20 | 84 |
| 10 | 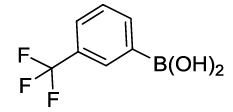 |
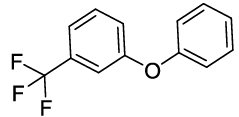 |
30 | 76 |
Table 2: Synthesized diaryl ethers derivatives (3a-j)
Conclusion
We have developed a general, simple and efficient method for the Pd(OAc)2-catalyzed for Chan–Lam coupling for the synthesis of aryl ethers derivatives. The formation of desired products proceeded well under 2'-bipyridine ligand and aerobic conditions with high efficiency and good optimum conditions for Chan–Lam coupling of phenol with and aryl boronic acid.
References
- G. Evano, N. Blanchard, M. Toumi, Chem. Rev., 2008, 108(8), 3054-3131.
- C.H. Burgos, T.E. Barder, X. Huang, S.L. Buchwald, Angewandte Chemie Int. Edn., 2006, 45(26), 4321-4326.
- F. Ullmann, P. Sponagel, Berichte der deutschen chemischenGesellschaf., 1905, 38(2), 2211-2212.
- D.M.T. Chan, K.L. Monaco, R.P. Wang, M.P. Winters, Tetrahedron Lett., 1998, 39, 2933-2936.
- P.Y.S. Lam, C.G. Clark, S. Saubern, J. Adams, M.P. Winters, D.M.T. Chan, A. Combs, Tetrahedron Lett., 1998, 39, 2941-2944.
- D.A. Evans, J.L. Katz, T.R. West, Tetrahedron Lett., 1998, 39(19), 2937-2940.
- D.M.T. Chan, K.L. Monaco, R.P. Wang, M.P. Winters, Tetrahedron Lett., 1998, 39(19), 2933-2936.
- L. Zhang, G. Zhang, M. Zhang, J. Cheng, J. Org. Chem., 2010, 75(21), 7472-7474.
- B. Sreedhar, G.T. Venkanna, K.B. Shiva Kumar, V. Balasubrahmanyam, Synthesis., 2008, 5, 795-799.
- K.S. Rao, T.S. Wu, Tetrahedron.,2012, 68,7735-7754.
- C. Fischer, B. Koenig, Beilstein J. Org. Chem.,2011, 7,59-74.
- B.K. Singh, C.V. Stevens, D.R.J. Acke, V.S. Parmar, E.V. Van der Eycken, Tetrahedron Lett.,2009, 50,15-18.
- B.N. Reddy, C.R. Rani, S.M. Reddy, M. Pathak, Research on chemical intermediates., 2016, 42(10), 7533-7549.